Chapter 4- Reaction Rates
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
what is activation energy?
the energy needed for a reaction to happen
activation energy is used to break bonds in the reactants before new bonds can form in the products
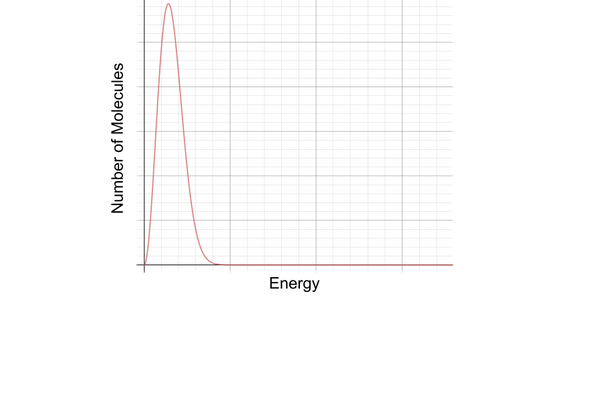
what is the maxwell-boltzmann distribution of energies?
a graph showing the energy distribution of all the molecules in a gas
the number of molecules is on the y-axis
the kinetic energy of the molecules is on the x-axis
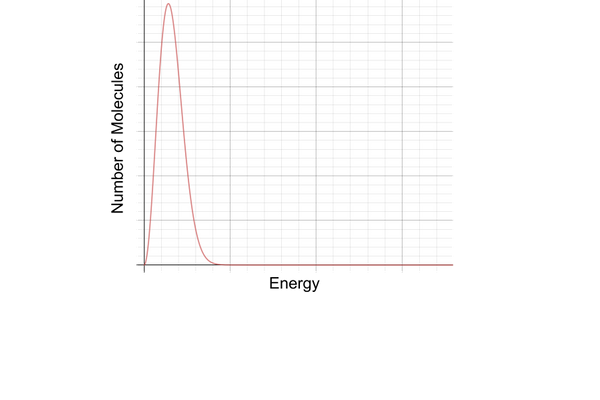
maxwell- boltzmann distribution: why does the curve pass through the origin?
because the molecules have zero energy
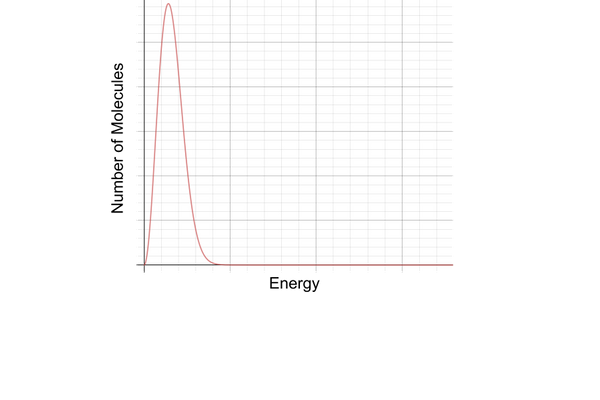
maxwell- boltzmann distribution: why is there a peak in the middle?
it represents the most likely energy of any molecule
there are more molecules with this energy than with any other energy
maxwell-boltzmann distribution: what is the mean energy?
it is to the right of the peak, and it divides the area below the graph into 2 equal halves
maxwell- boltzmann distribution: what is the area under the graph showing?
the total number of molecules
how is the curve asymptotic?
it doesn’t reach the x-axis
this is because there can be no maximum value for the kinetic energy of a molecule
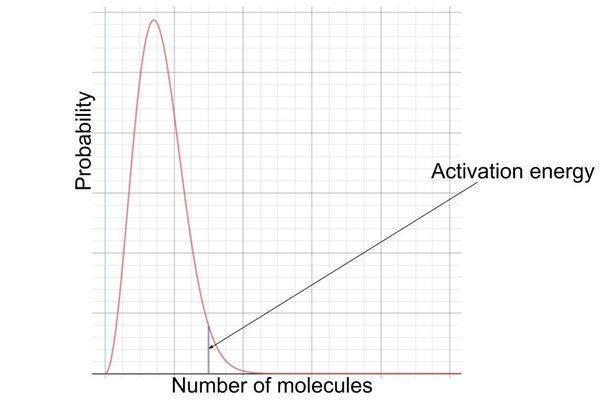
maxwell- boltzmann distribution: where is the activation energy marked?
this allows us to view the number of molecules with the energy to react
on the left hand side of the activation energy mark, molecules have less energy than the activation energy and so they can’t react
on the right hand side, molecules have more energy than the activation energy and so they can react
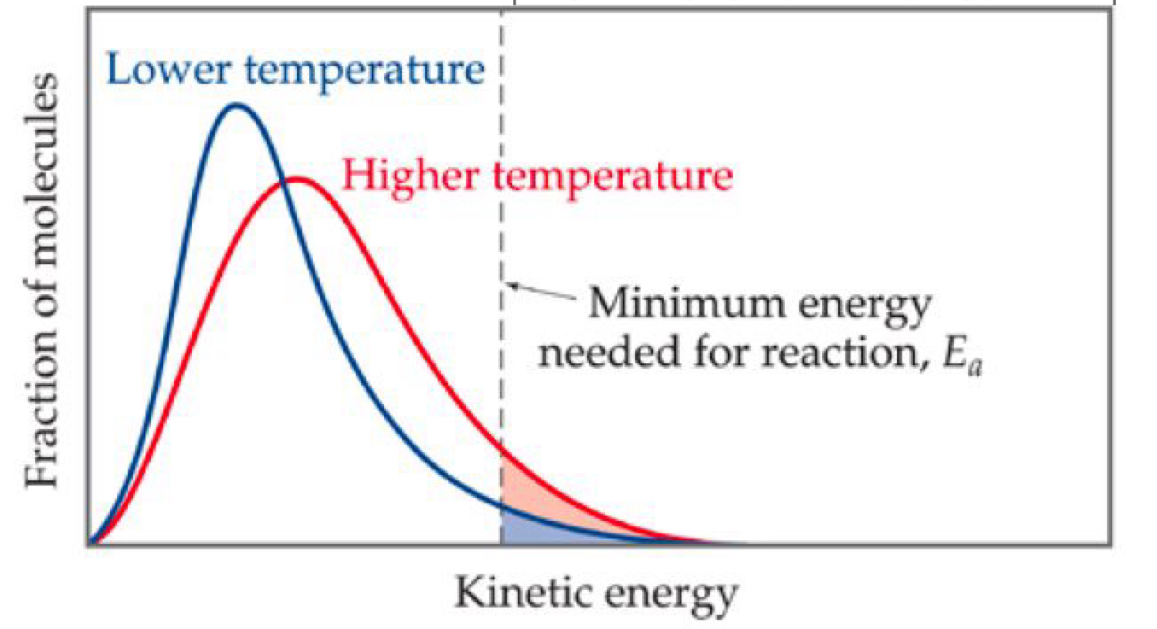
maxwell- boltzmann distribution: effect of heating on the maxwell-boltzmann distribution of energies?
it raises the average energy but lowers the peak of the graph
it shifts to the right because a greater proportion of molecules have greater kinetic energy
therefore, a greater proportion of molecules will have energy greater than or equal to the activation energy
maxwell-boltzmann distribution: effect of concentration, pressure and surface area?
increasing the conc, pressure or surface area of reactants make successful collisions occur more frequently
however, they don’t change the energy of the individual particles
therefore, shape of the maxwell-boltzmann distribution doesn’t change
what are the 3 main methods for changing reaction rates?
changing temperature
changing concentration
changing pressure
how does increasing concentration increase rate of reaction?
increasing concentration increases number of collisions that happen between the reactants
there will be more successful collsions
therefore, the rate will increase
how does changing pressure increase rate of reaction?
increasing pressure reduces volume of the gas
this means molecules are closer together
if the molecules are closer together, they will collide more often
therefore, more successful collisions and the rate will increase
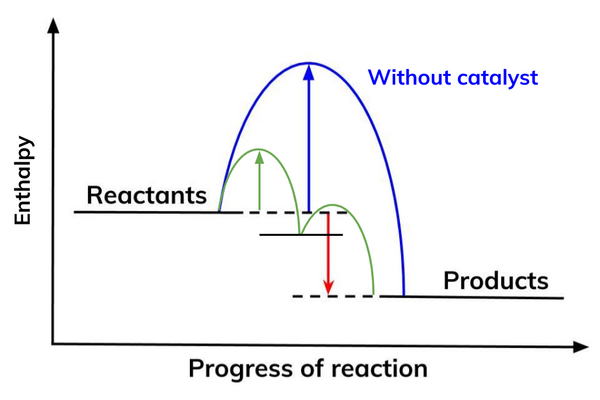
how does adding a catalyst increase the rate of reaction?
it offers an alternative pathway with lower activation energy
therefore, a greater proportion of molecules will have the energy greater than the activation energy
so, there will be more successful collisions
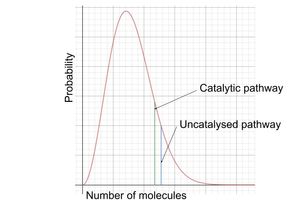
catalysts on a maxwell-boltzmann graph?
catalytic pathway is shifted to the left and can be used by more molecules
however, curve is unchanged in shape
more molecules have enough energy to react via the catalysed pathway
why is it necessary to heat the calcium carbonate strongly to achieve decomposition?
to overcome activation energy
reaction is endothermic
to break bonds