Chem learning check 1.1 (atomic models,indigous plants and chem careers)
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Atomos Model
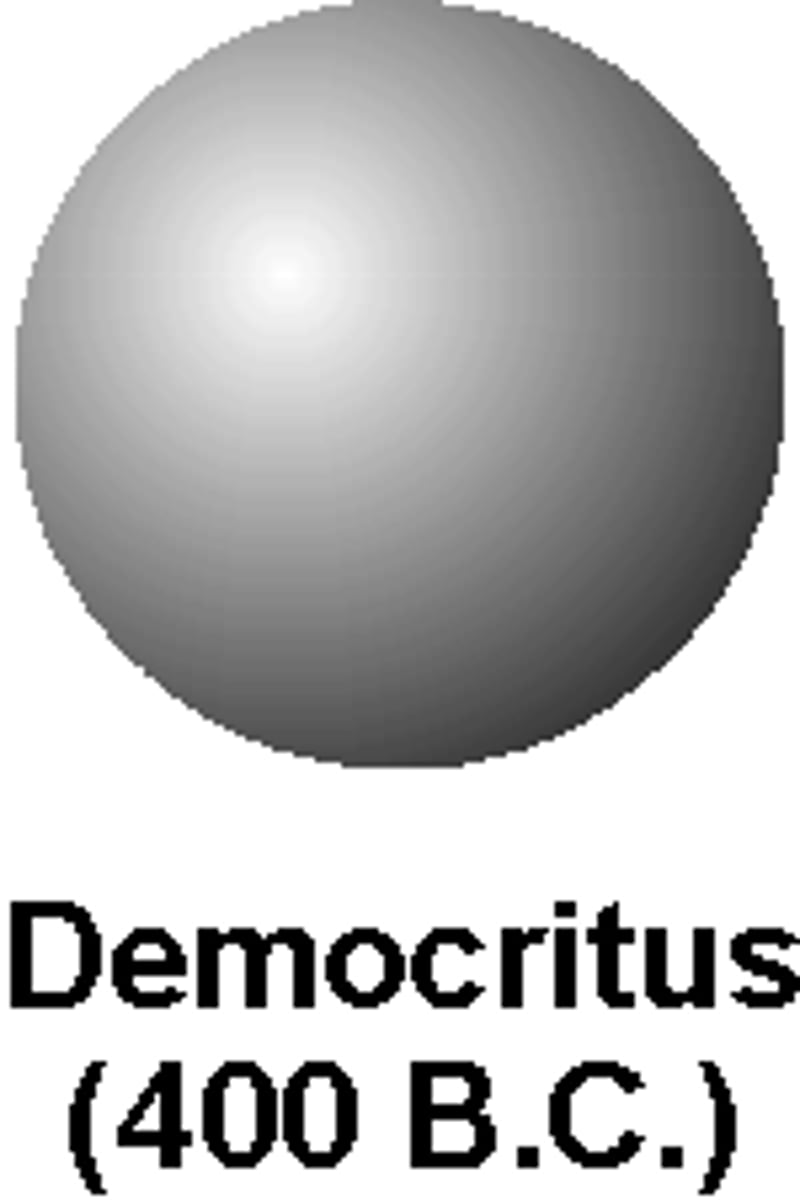
Created by democritus 400 B.C- suggested that all matter is composed of tiny uncuttable or invisible units
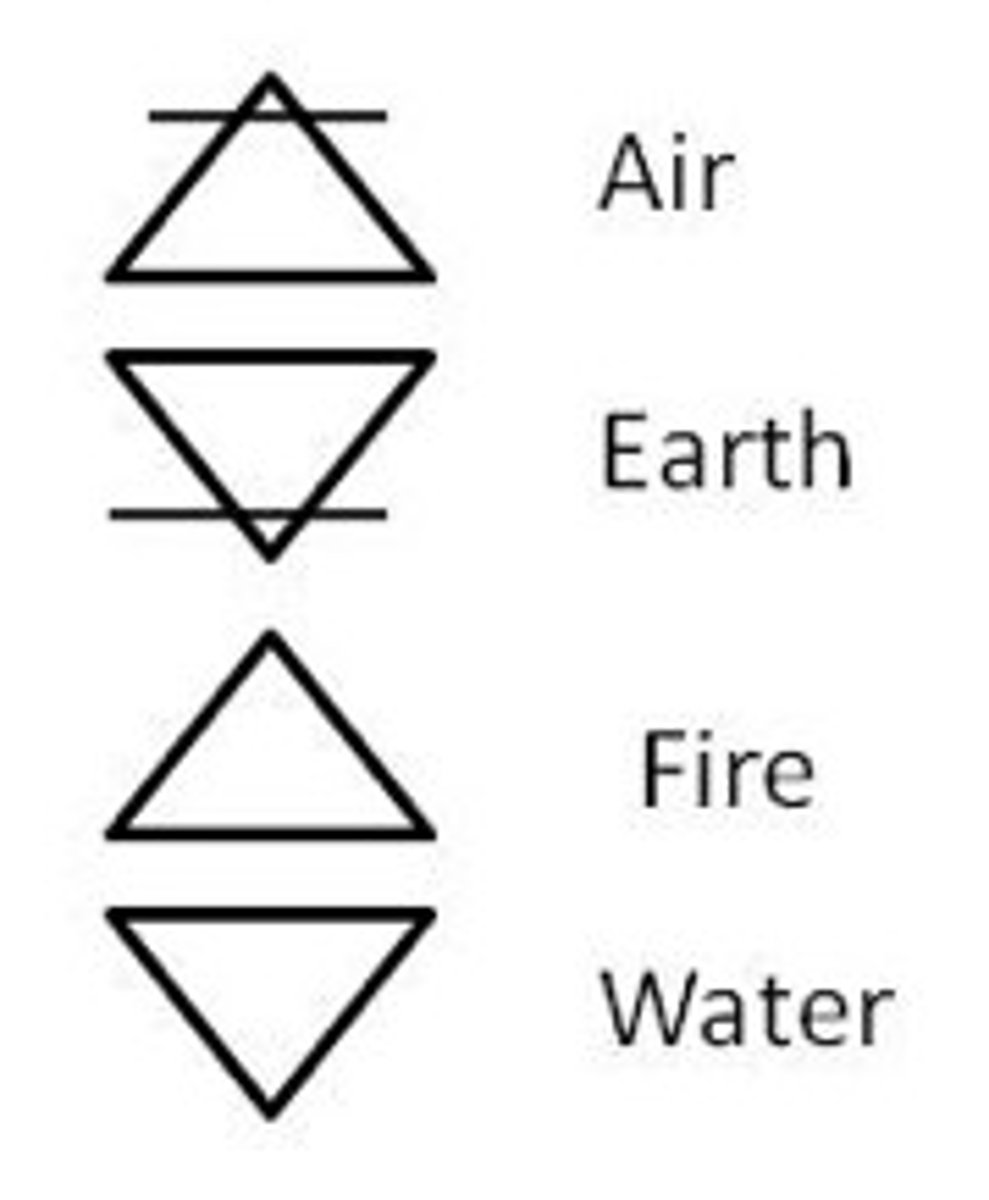
earth, air, water, fire
Created by Aristotle 350 B.C- believed in the 4 elements-- felt that regardless of the # of times you cut form of matter in half, you would always have a smaller piece of matter. All matter is created by the 4 elements
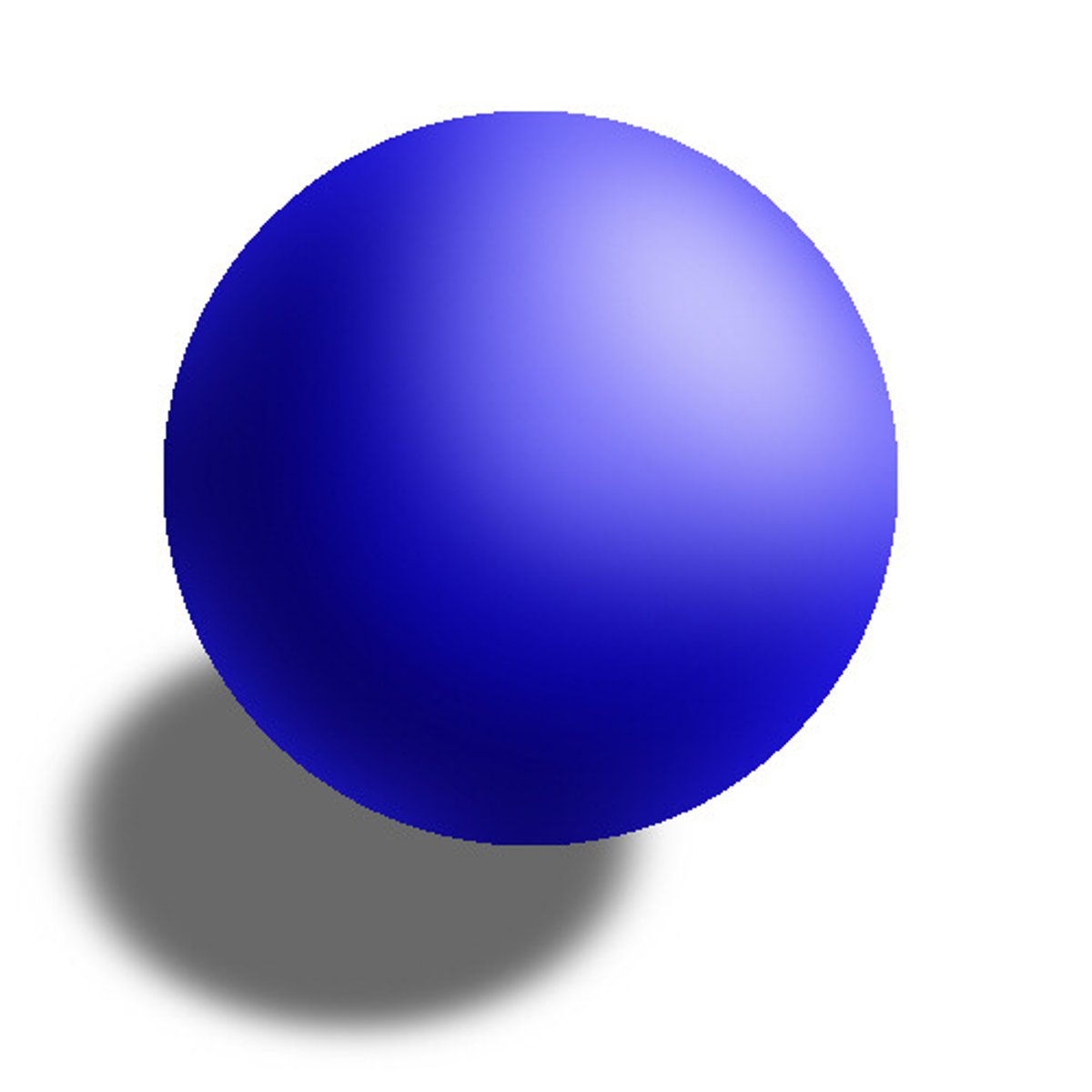
Solid sphere model
Dalton- atoms are tiny solid,indivisible particles. different from democritus because he had evidence (law of conservation mass)
Of Datons 5 claims what was correct
3.Atoms of different elements differ in their physical and chemical properties
4.Atoms of dif elements combine in simple,whole # ratios to form compounds
5.in chem reactions,atoms are combined,separated,or rearranged, but never created,destroyed or changed
Which claims where incorrect
1.All elements are composed of tiny indivisible particles called atoms that cannot be broken down further
2.Atoms of a given element are all similar in their physical and chem properties
Plum Pudding Model
J.J Thomsons model of an atom, in which he thought electrons were randomly distributed within a positively charged cloud.
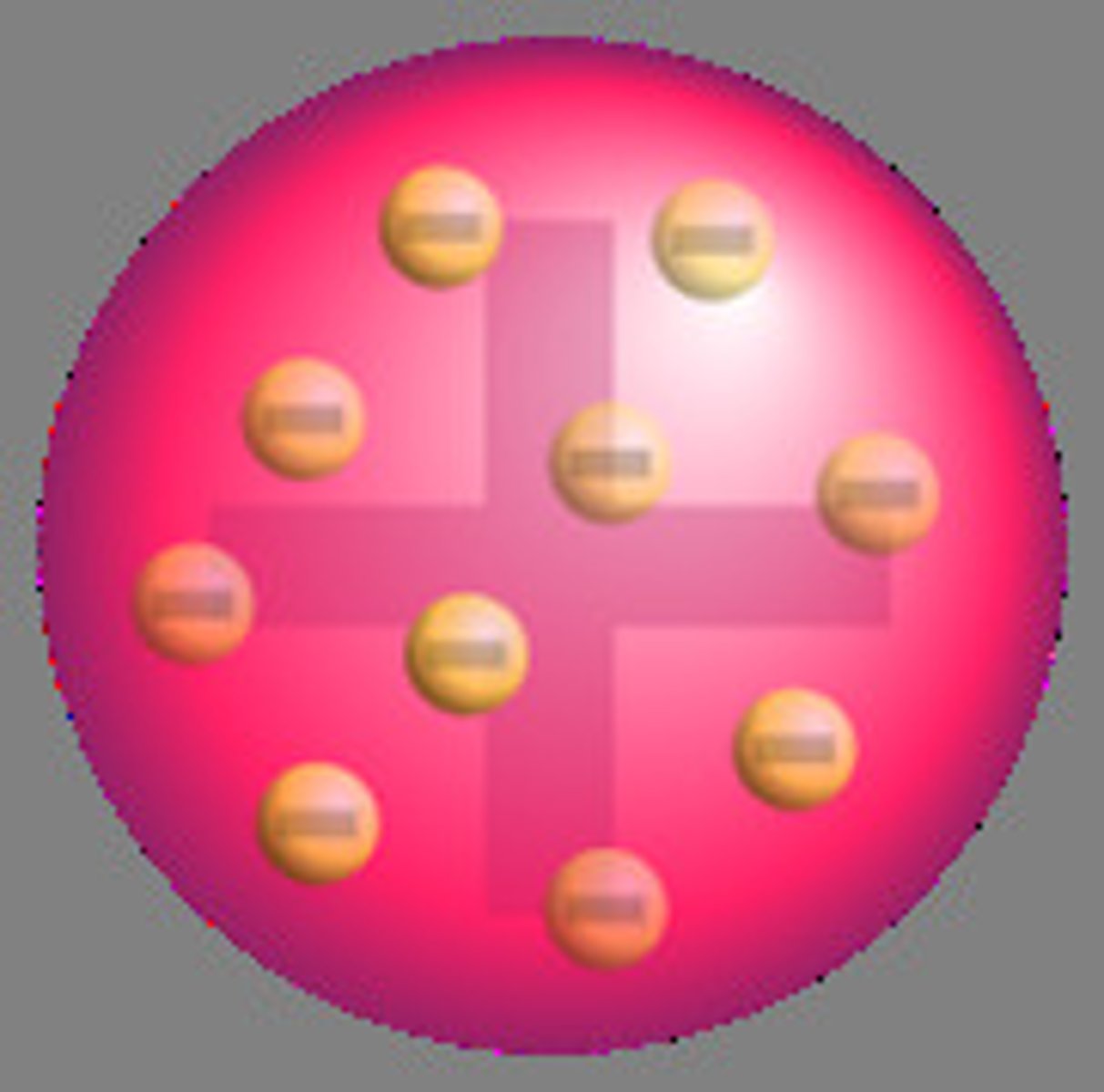
How did he find this
J.J. Thomson's cathode ray tube experiment (1897) showed that atoms contain tiny, negatively charged electrons. He proposed the plum pudding model, where electrons were embedded in a positively charged "pudding." This model was later disproven by Rutherford's gold foil experiment.
Nuclear model
Ernest Rutherfords Model of the atom with a nucleus containing protons and neutrons and with electrons in the space outside the nucleus. He is credited for discovering the nucleus
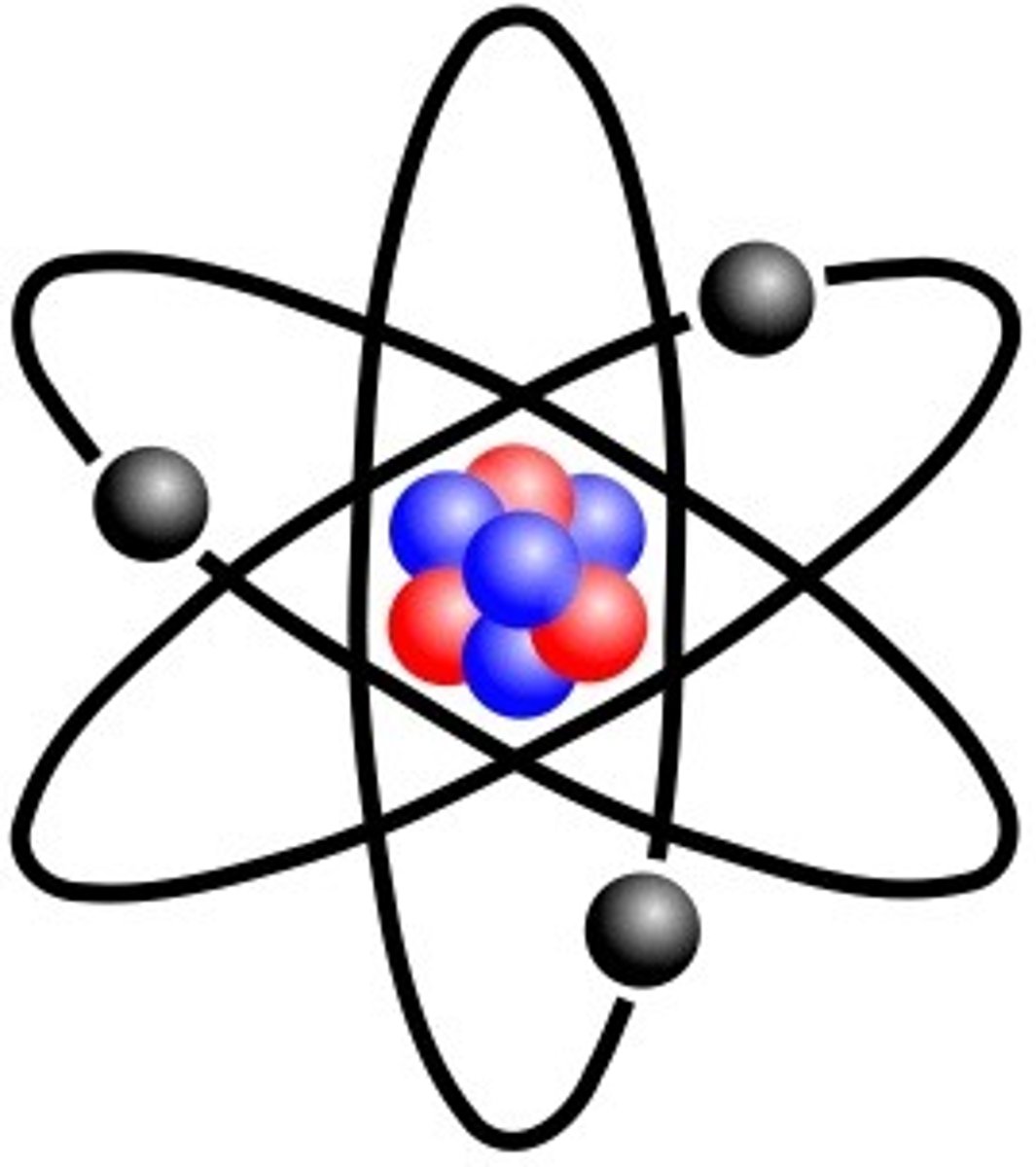
What experiment helped him find this
gold foil experiment (1909) involved shooting alpha particles at a thin gold foil. Most passed through, but some deflected or bounced back, suggesting that atoms have a small, dense, positively charged nucleus instead of a uniform spread of charge. This led to the nuclear model of the atom, replacing the plum pudding model.
Planetary model
Niels Bohr electrons move around the nucleus in fixed, circular orbits
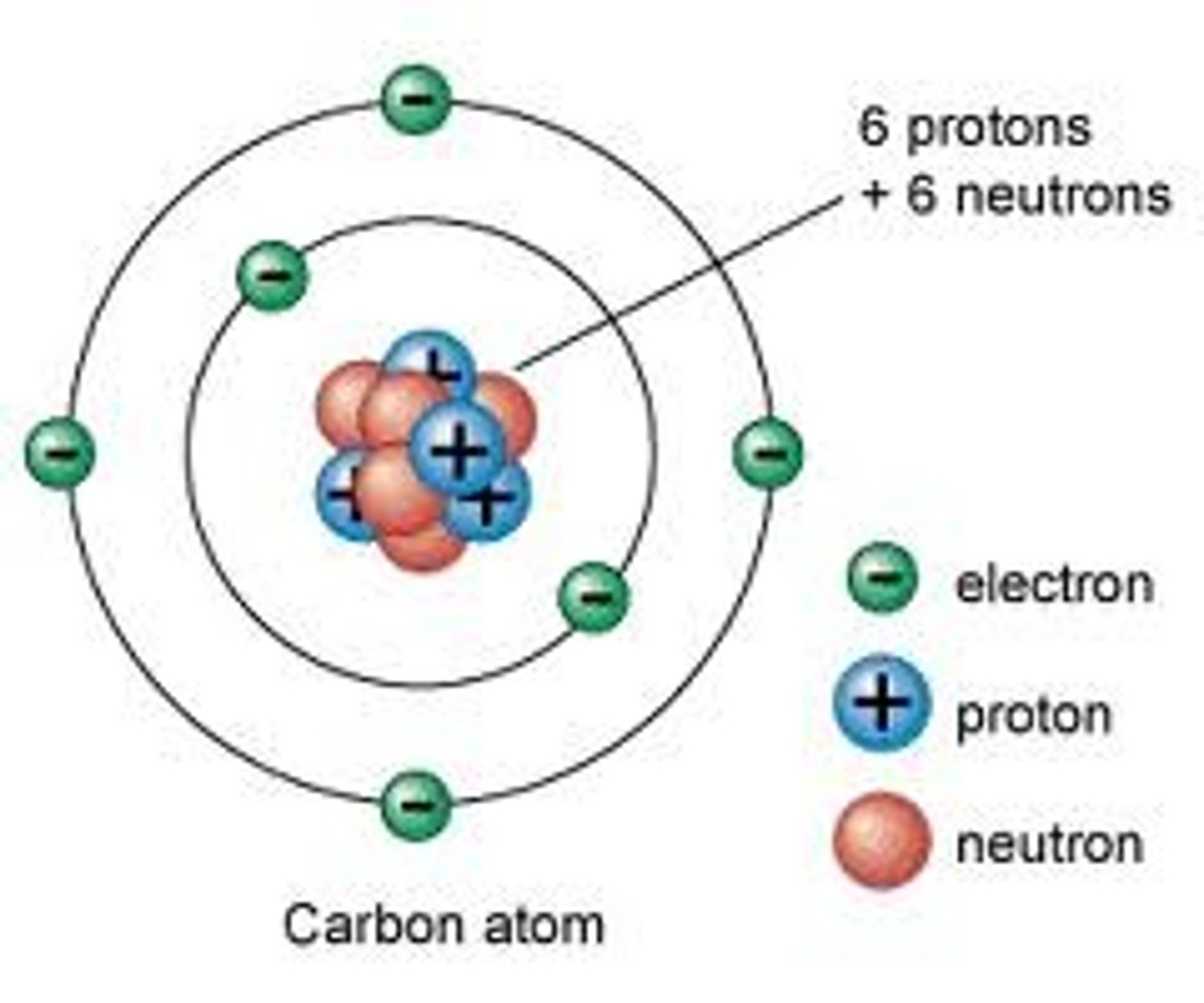
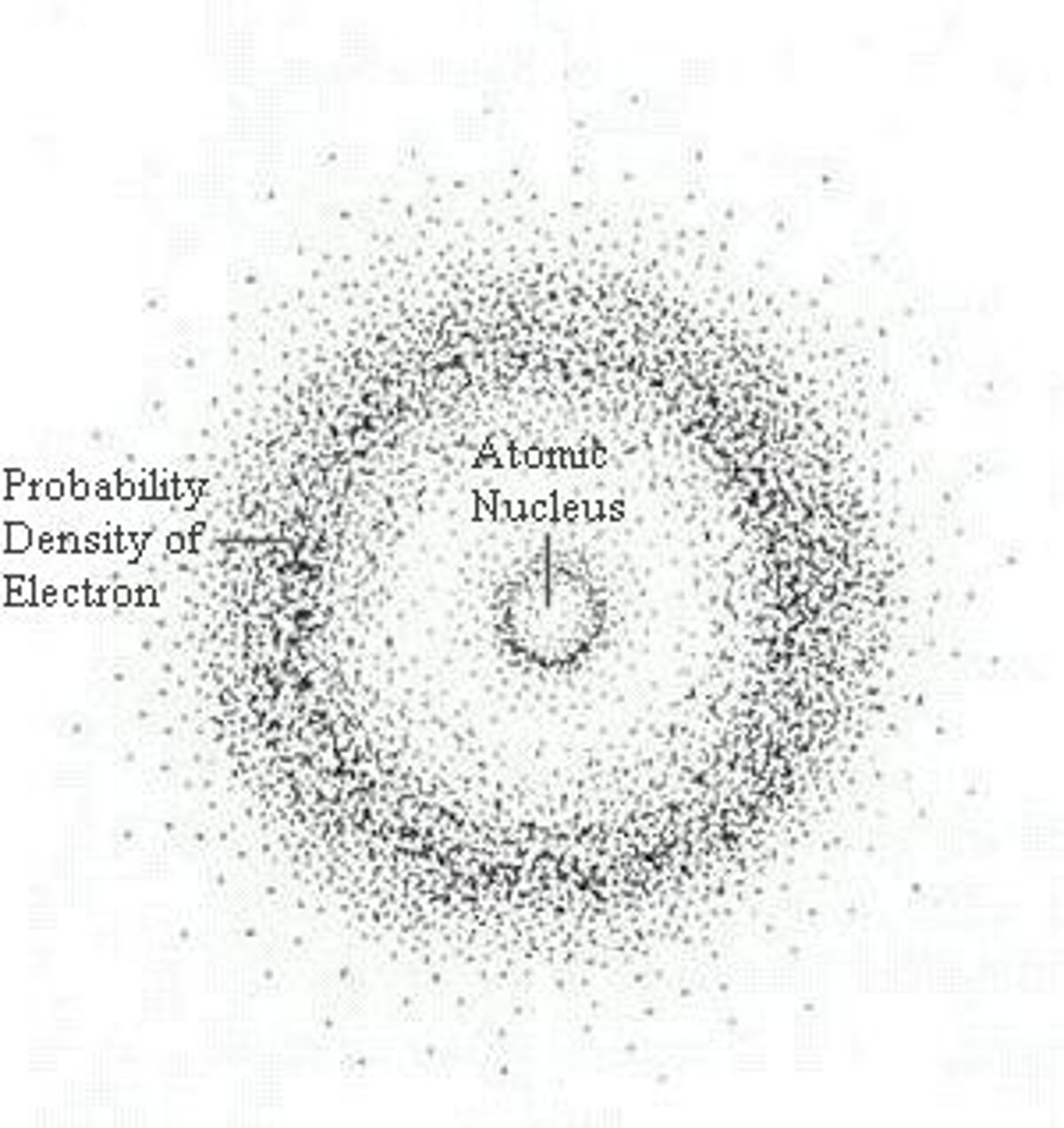
The quantum model
made by Erwin Schrödinger current atomic model in which a tiny, dense atomic nucleus is surrounded by a "cloud" of electrons occupying three-dimensional orbitals according to their energies