PCOL L4 GPCR Part 1
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
intracellular receptors
nuclear/steroid hormone receptors, soluble cellular enzymes, structural proteins, nucleic acids
intracellular transcription factors
lipophilic chemicals which diffuse across the plasma membrane and bind receptors in cytosol or in nucleus
activated receptors bind dna and start gene transcription
cell-surface receptors
ligand and voltage gated ion channels, GPCRs, receptor tyrosin kinases
how many subunits does nicotinic acetylcholine receptor have
5
what binds to to nicotinic acetylcholine receptor
ACh, a neurotransmitter, binds two alpha subunits of the nicotinic receptor
what happens after ACh binds to nicotinic acetylcholine receptor
channel opens and allows Na+ to pass
what are the three main parts of GPCR
transmembrane receptor, G protein, effector
what are the subunits of G proteins
3 subunits: alpha, beta, gamma
nucleotide mediators
extracellular nucleotides that act as signaling molecules (GTP and GDP)
act as molecular on/off switches for intracellular signaling
what is an enzymatic cytosolic domain
the intracellular portion of a transmembrane receptor that has catalytic (enzyme) activity
ie. tyrosine kinase, serine/threonine kinase, tyrosine phosphotase
what defines a transmembrane receptor
receptor that spans the cell membrane to cause signaling for intracellular responses
kinases
phosphorylation: add phosphate groups (PO4-) to proteins
act as the ON switch for signaling
What does tyrosine kinase do
phosphorylates tyrosine residues on target proteins
what is a receptor tyrosine kinase (RTK)
transmembrane receptor with intrinsic tyrosine kinase activity in its cytosolic domain
usually dimerize to activate the kinase for autophosphorylation (receptor phosphorylates itself after activation)
what residues are phosphorylated by serine/threonine kinases
serine and threonine residues
what is the role of phosphatases in signaling pathways
they remove phosphate groups to turn signaling OFF
what enzyme activity converts GTP to cGMP
guanylyl cyclase
which enzyme class is a major target in cancer therapy? what drug targets it?
tyrosine kinase, imatinib
signal transduction
process by which a cell converts information (signal) from one form to another
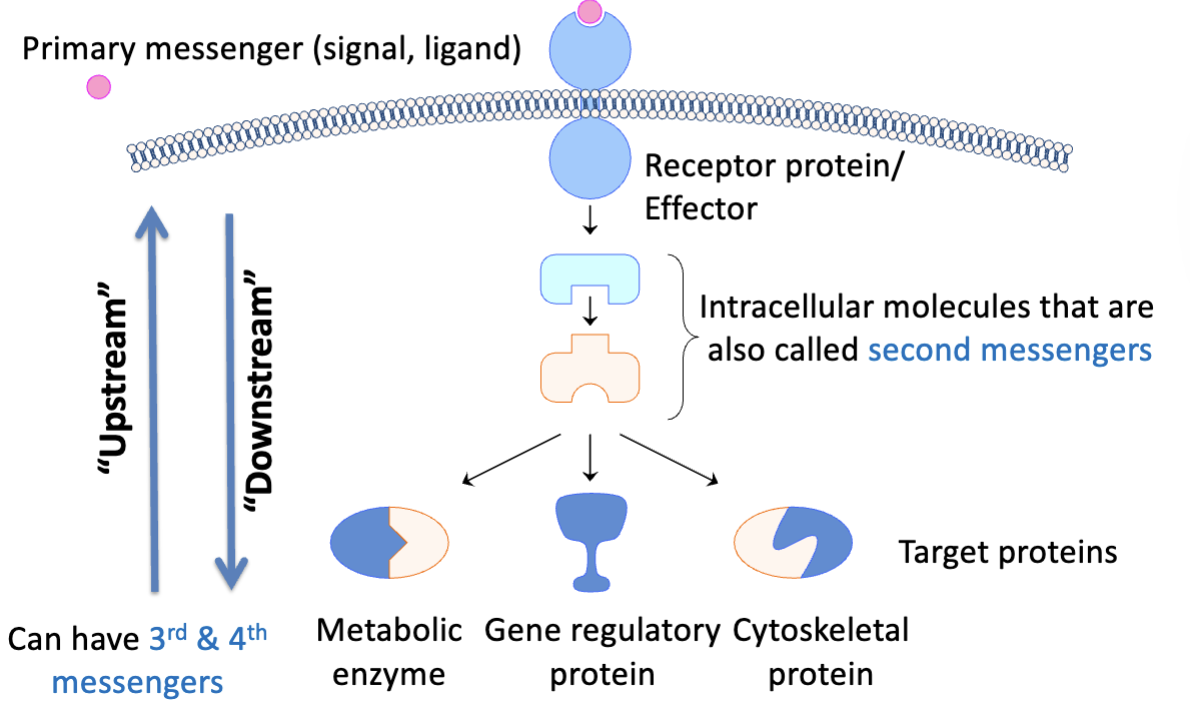
general steps of signal transduction
generation of signal (distant or local)
accumulation of signal at target site
binding of signal to effector protein (often a receptor)
induction of signaling event (conformation changes in effector, propogation of signal, can be fast or slow)
termination of signaling event
primary messengers
often extracellular ligands that bind and activate receptors (some intracellular receptors) and can have multiple functions
act on cells they were released from (autocrine), nearby cells (paracrine), distant cells (endocrine)
what are hormones and how do they reach their target organs
they are primary messengers released in small amounts from one site that travel thru bloodstream to act on distant target organs (endocrine signaling)
why must hormone receptors have high affinity?
they are diluted in circulation and bound to carrier proteins, resulting in low free concentrations requiring high-affinity receptors
growth factors
primary messengers that stimulate proliferation and also have high affinity receptors, tend to act locally and action can last days
cytokines
primary messengers that work on immune and other cell types to elicit biological responses
chemokines
cytokines that activate and recruit inflammatory cells by chemotaxis
vasoactive agents
primary messengers released at injury site to allow vasodilation and vascular permeability to recruit leukocytes/phagocytes
neurotransmitters
primary messengers released from presynaptic cells into the synaptic cleft that diffuse and bind receptors on presynaptic and postsynaptic cells
can be excitatory (glutamate) or inhibitory (gaba and glycine)
secondary messengers
intracellular molecules formed or activated in response to activation of receptors, often transmit signals from outside to inside of cell
ie. cAMP,GMP, Ca2+, DAG
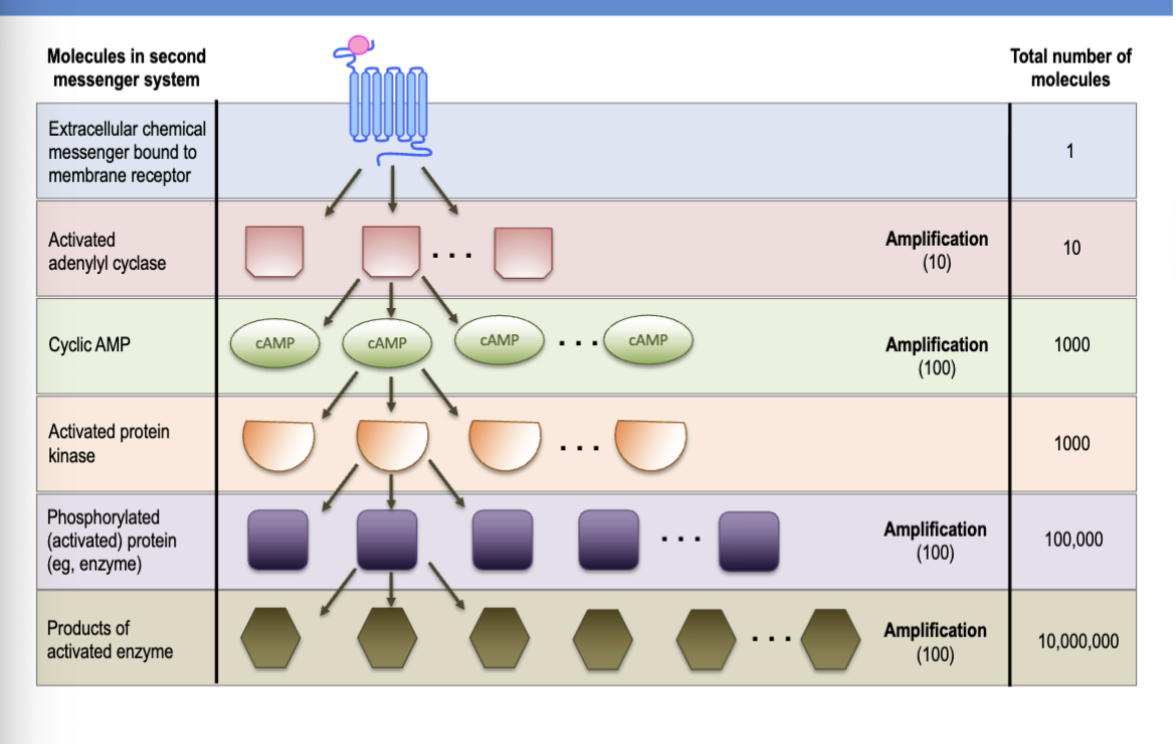
signal amplification
binding of one ligand to receptor will activate multiple second messengers(adenyl cyclase → cAMP) which can activate multiple kinases which can then phosphorylate ode proteins leading to an amplified response
7 transmembrane receptors
single plypeptide chain traverses plasma membrane seven times (3 intracellular and 3 extracellular loops)
variety of ligands (growth factors, glucagon, epinephrine, prostaglandins, ACh, opioid peptides, etc
types of ligand-GPCR interactions
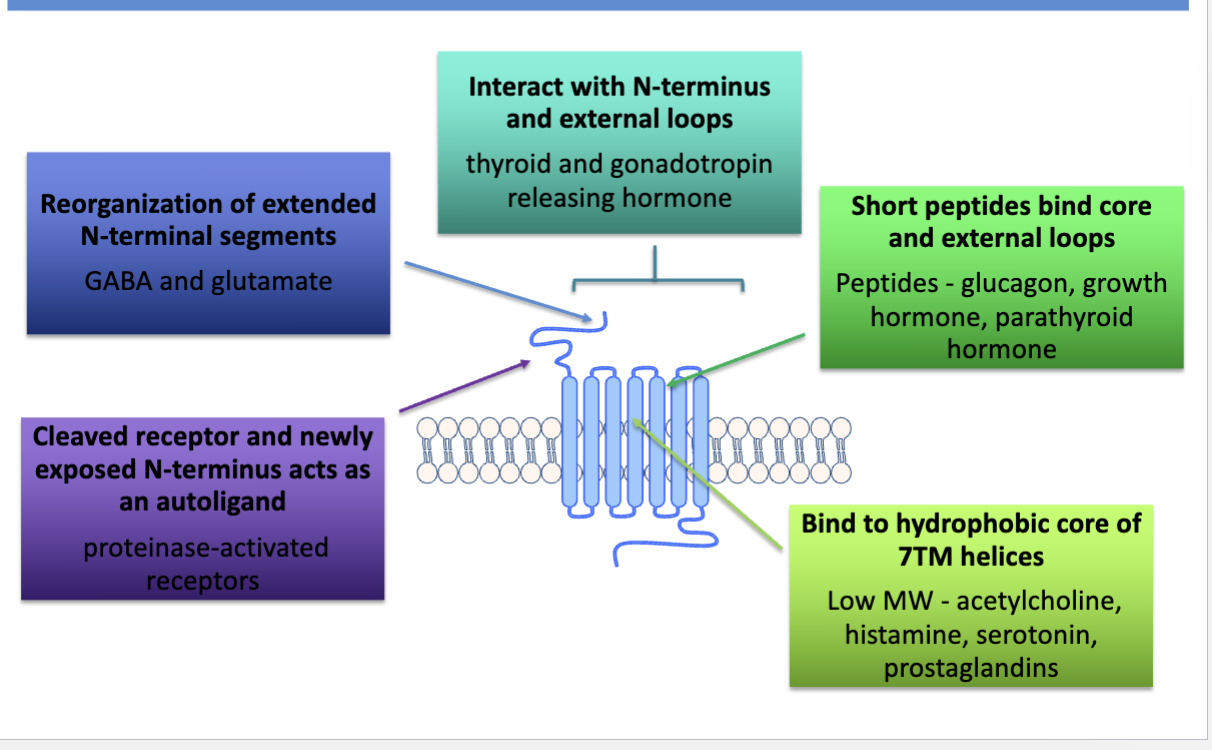
what are some processes and examples of GPCRs
vision, olfaction, cns, immune system, digestive system
ie. beta adrenergic, thyrotropin, glucagon and some types of dopamine and serotonin receptors
what are the 4 nucleotides and their functions
CTP: phospholipid biosynthesis
UTP: polysaccharide assembly
ATP: metabolism & cell activity
GTP: Receptor singling cofactor
what are g-proteins
gaunine nucleotide-binding receptors (also known as molecular switches or effectors)
they are the intermediary proteins in signal transduction
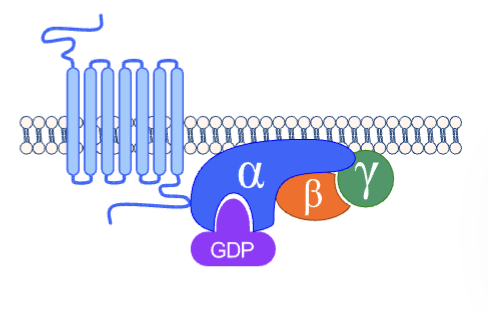
where are g-proteins found
along the inner surface of plasma membrane
when are g-proteins active/inactive
bound to gdp is inactive, bound to gtp is active
what is the difference between alpha, beta and gamma subunits of g-proteins
alpha subunit has the nucleotide binding site
beta and gamma act as 1 entity and are anchored to membrane by lipids
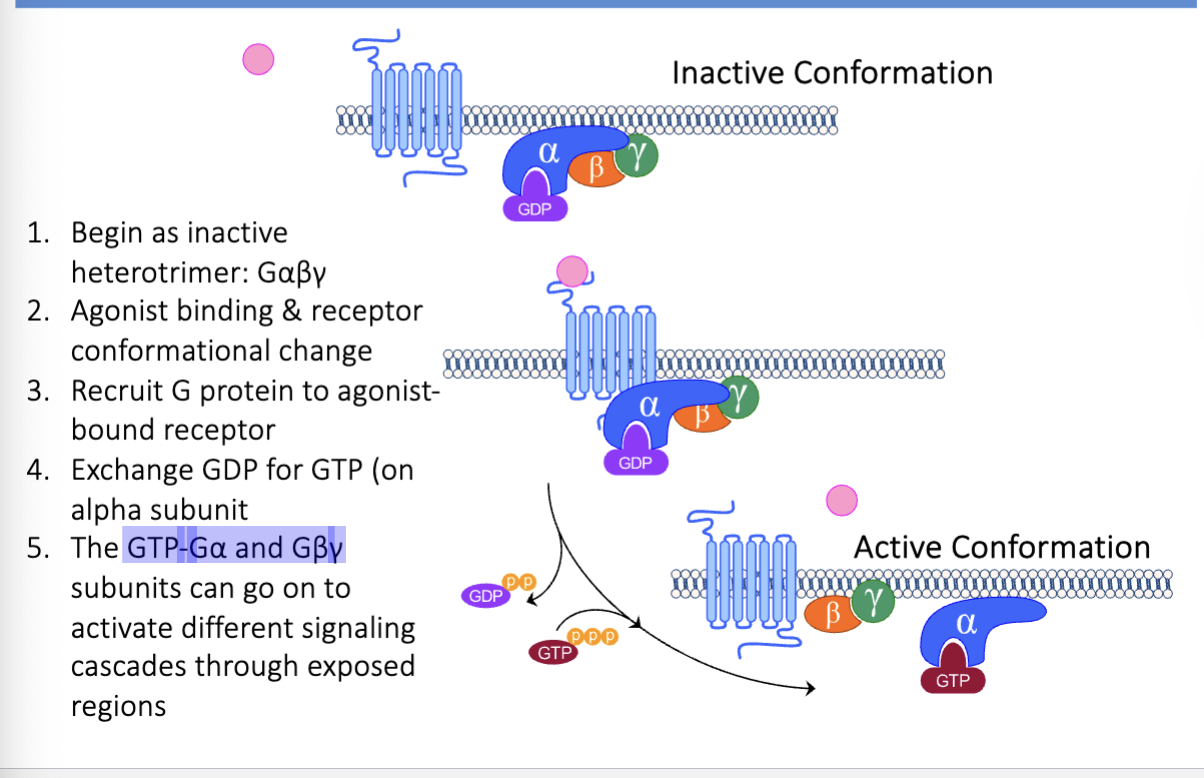
what are the g-protein activation steps
start as inactive heterotrimer: Gαβγ
agonist binding & receptor conformational change
recruit g protein to agonist-bound receptor
exchange gdp for gtp (on alpha subunit)
GTP-Gα and Gβγ subunits dissociate and activate different signaling cascades
what are the steps in g-protein inactivation
Gα subunit comes into contact with effector enzyme → GTPase activated
Gα subunit hydrollyze GTP to GDP
Gα and Gβγ reassociate
inactive form
why is the signal still amplified during g-protein inactivation
because hyrolysis of GTP is SLOW and allows stimulation of a number of effector molecules
what do the c-terminus and n-terminus on alpha subunit interact with
c-terminus interacts with receptors
n-terminus interacts with βγ subunit
after binding or gtp and dissociation of βγ dimer what happens to the α-subunit
surface of α-subunit is shownto effector molecule to interact
why is there so much diversity of heterotrimeric g proteins
because the α-subunit has different types
ie. universal expression (αs, αi, α11, αq)
ie. sensory (αt,αolf) differs from neurons (az)
what does the identity of the βγ dimer contribute to
coupling of G-proteins to particular receptors, localization, coupling and deactivation of a-subunit, reduce tendency of GDP to dissociate from a-subunit
how do βγ subunits act as signaling proteins
increase K+ channel activity, decrease Ca2+ channel activity
what enzyme catylyzes conversion of ATP to cAMP
adenylyl cyclase
what enzyme catalyzes the breakdown of cAMP to 5’-AMP (adenylate)
phosphodiesterase
how is adenylyl cyclase regulated
activated by certain Gα subtypes, forskolin, Ca2+-calmodulin by
deactivated by certain Gα subtypes
Gαs
stimulates production of cAMP from ATP by activating adenylyl cyclase
Gαi
inhibits production of cAMP from ATP by decreasing adenylyl cyclase
Gαq
increase phospholipase C activity
protein kinase function
turn enzymes on or off by phosphorylation
what exactly is added during phosphorylation
kinase add negatively charged phosphate groups (PO4-) to amino acids with an -OH group (hyrdoxyamino acids: serine, threonine, and sometimes tyrosine)
why do kinases specifically add to serines, threonines and tyrosines
because they are neutral, exposed on the protein surface and location at interfaces between protein subunits
protein kinase A (PKA)
soluble kinase with 2 catalytic and 2 regulatory domains (inactive tetramer form) that phosphorylates serine or threonine residues to produce multiple biological effects, has tissue-specific regulatory subunits,
activation of PKA
inactive tetramer binds to cAMP on regulatory subunits and detaches from active catalytic subunits
cAMP response element binding protein (CREB)
interacts with DNA at the cAMP response element (CRE) after being phosphorylated and activated by PKA
CRE
cAMP response element, 8 base pair palindromic sequence targeted by CREB
why is binding of cAMP onto PKA “cooperative”
because the activation energy of first cAMP is bind PKA is greatest, making the binding of each consecutive cAMP easier with a lower activation energy