Important Hydrocarbon Functional Groups
1/9
Earn XP
Description and Tags
Flashcards focused on key hydrocarbon functional groups and their definitions.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Halide
A functional group consisting of a halogen atom (F, Cl, Br, I) attached to a carbon.
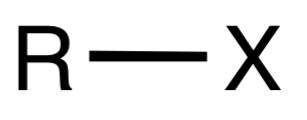
Alcohol
A functional group characterized by the presence of a hydroxyl group (-OH) attached to a carbon.

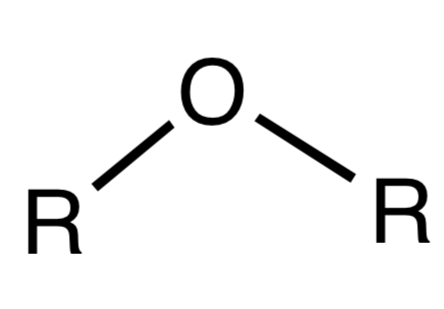
Ether
A functional group where an oxygen atom is bonded to two hydrocarbon groups.
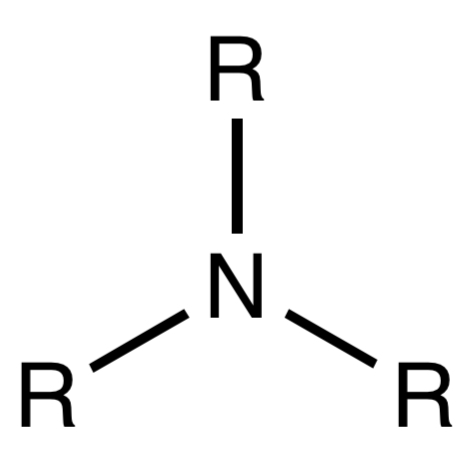
Amine
A functional group containing a nitrogen atom bonded to one or more hydrocarbons.
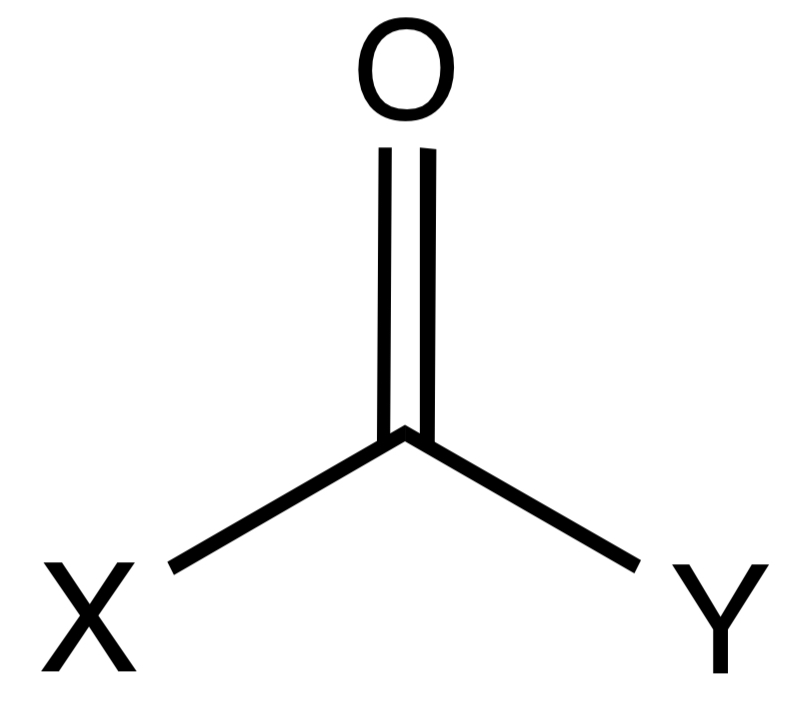
Carbonyl
A functional group composed of a carbon atom double bonded to an oxygen atom.
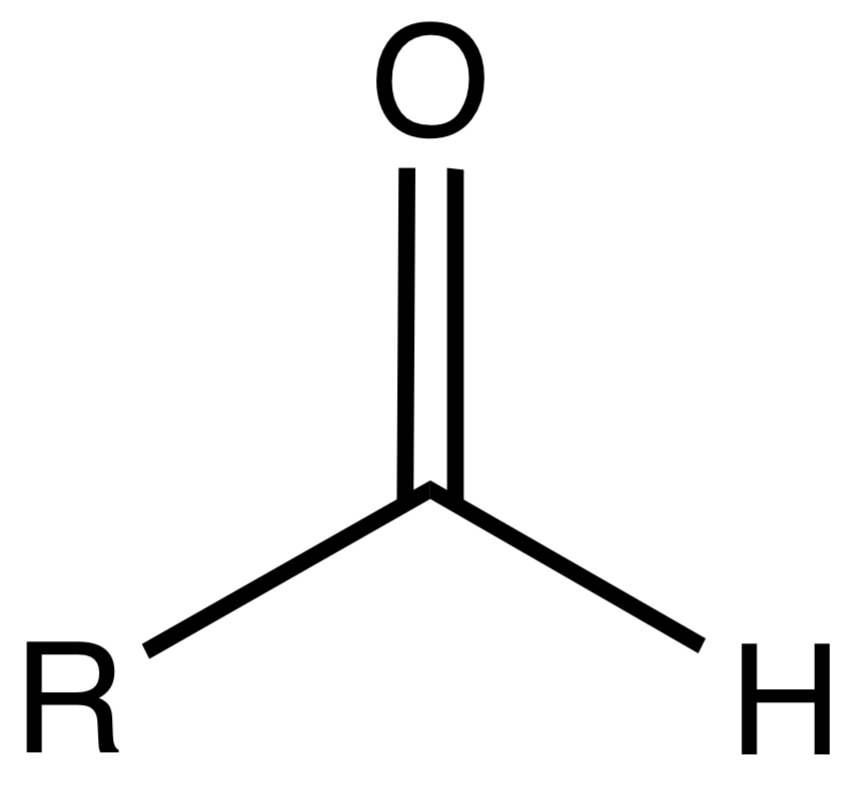
Aldehyde
An organic compound containing a carbonyl group (C=O) with at least one hydrogen atom attached.
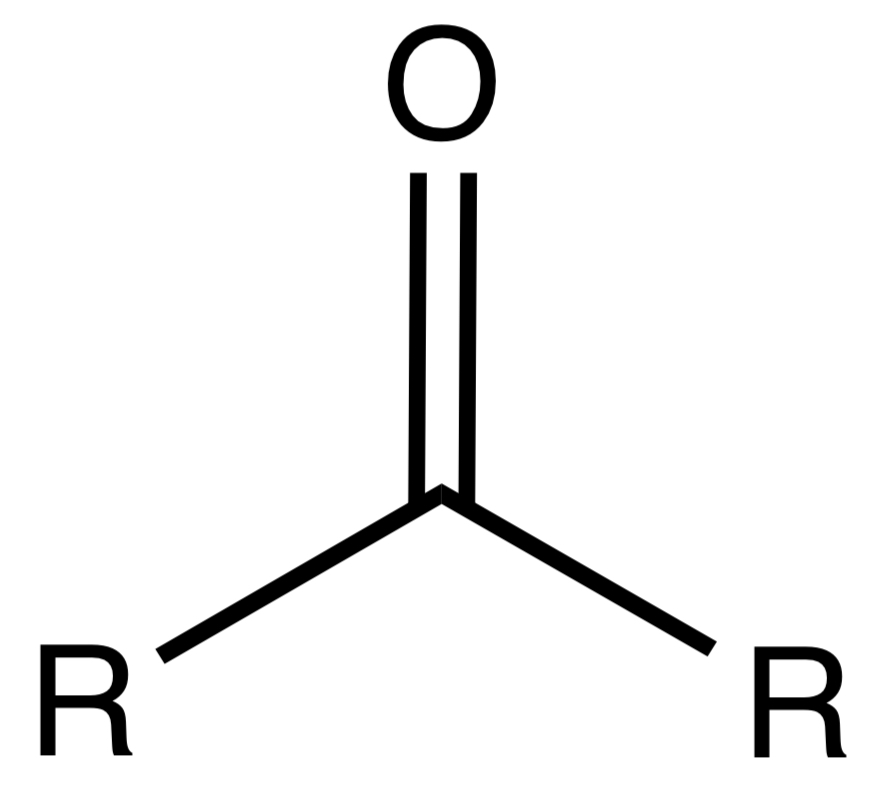
Ketone
An organic compound containing a carbonyl group (C=O) bonded to two carbon atoms.
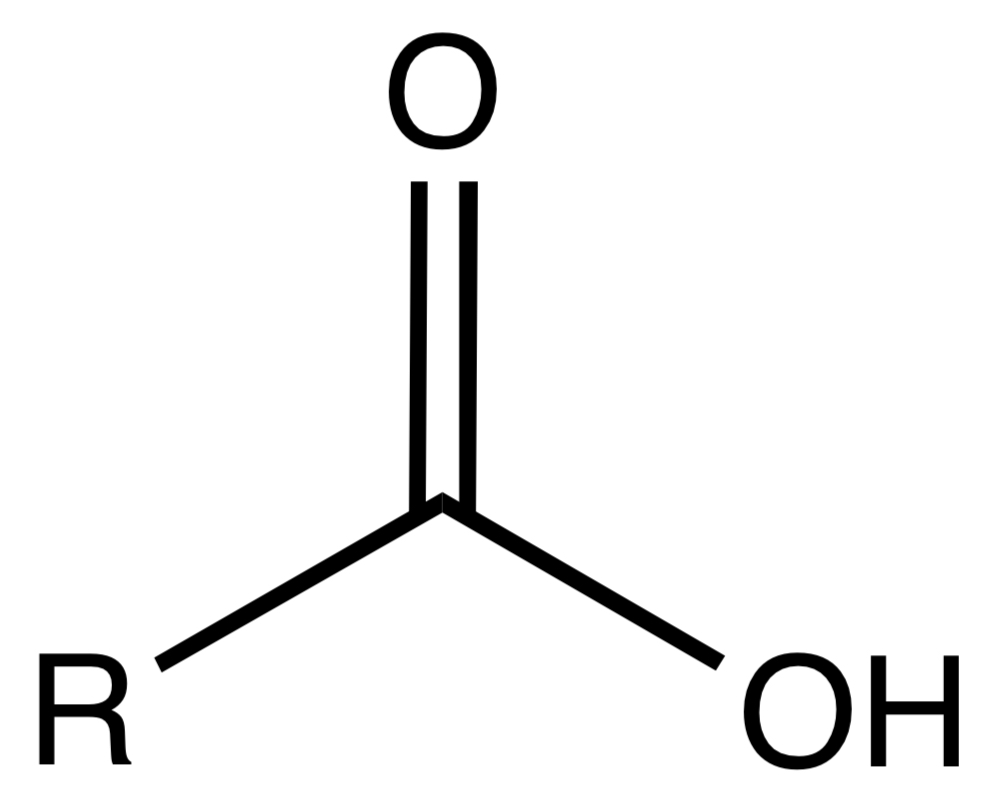
Carboxylic Acid
A functional group characterized by a carbonyl and a hydroxyl group (-COOH) attached to the same carbon.
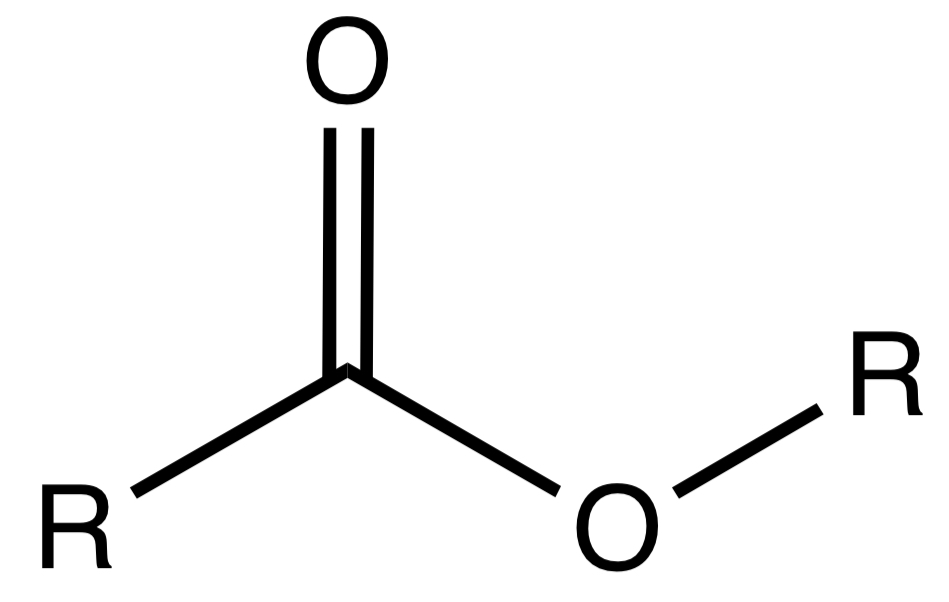
Ester
A derived functional group formed from the reaction of an alcohol and a carboxylic acid.
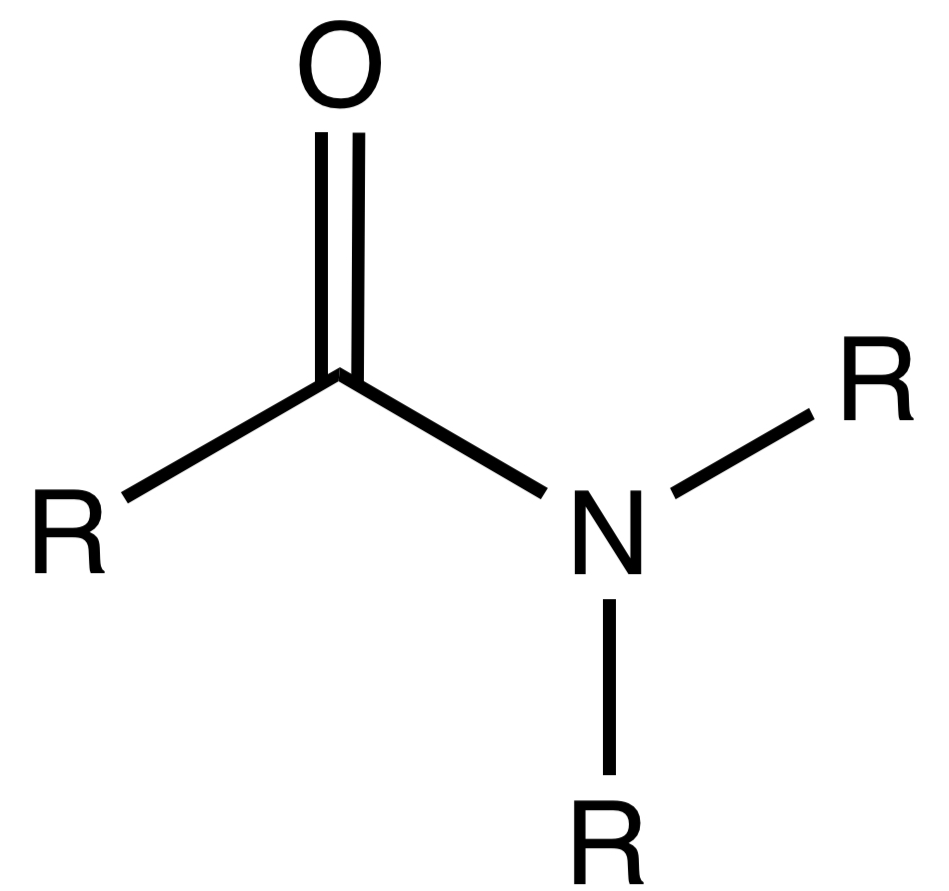
Amide
A functional group containing a carbonyl group (C=O) adjacent to a nitrogen atom.