Chemistry Final
1/13
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
How many valence electrons are needed to fulfill a full outer shell?
8
Entropy is a measure of randomness or disorder. Between a cold piece of solid iron and a hot piece of solid iron which one has more entropy?
Hot, Because the particles are moving around faster
What is the scientific notation for 3.0024 x 10-6
0.0000030024
Which type of system allows for energy, but not matter to transfer in or out.
Open, Closed, or Isolated
Closed, It can only exchange energy only energy with its surroundings, not matter.
Which particle is responsible for the atomic number of an element
Protons, The atomic number will match the number of protons.
How much mass and charge do neutrons have?
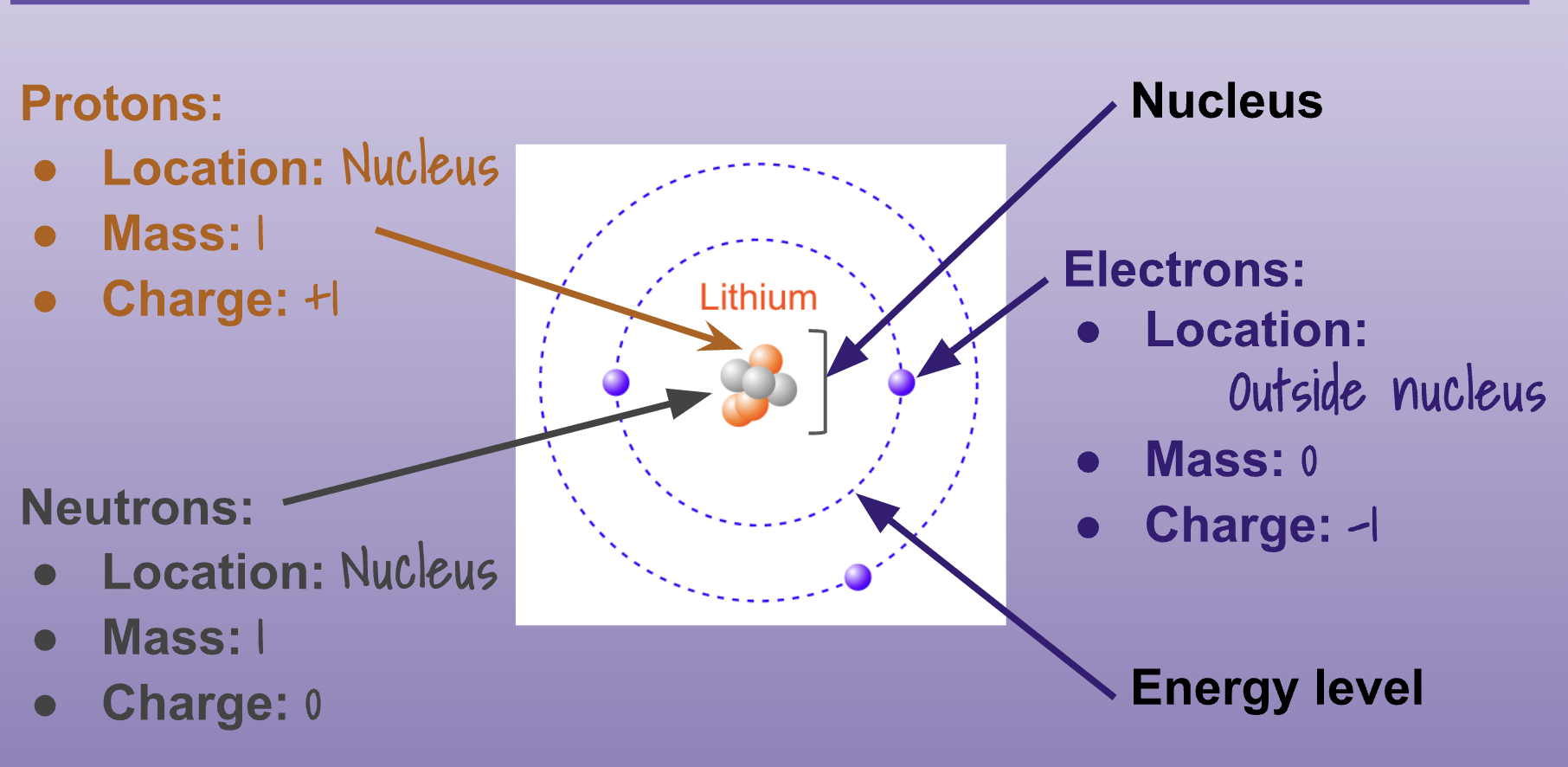
Mass 1, Charge 0
Which type of system does not allow for energy or matter to transfer in or out?
Isolated
What is the difference between a compound and mixture
Compounds are substances which can be formed by chemically combining two or more elements. Mixtures are substances that are formed by physically mixing two or more substances
An element has an atomic # of 30 and a mass # of 65 and a +1 charge. List the number of protons, neutrons, & electrons below:
Zinc+1- 65 p+ = _________ n0 = _________ e – = _________
p+ = 30 n0 =35 e – =29
Which point is where solid, liquid, and gas all exist?
A
B
C
A yay
How many Kilograms are 36500 grams?
36.5 Kilograms, because 1 Kilogram equals 1000 grams.
What is a compound?
a pure substance made of two or more elements that are chemically bonded
What is the formula for calculating density?
Density = mass/volume.
How does the boiling point of a liquid change as atmospheric pressure decreases
A. It increases
B. It decreases
B, Less “pushing down” of pressure so it requires less temp to boil