MCAT Organic Chemistry - Analyzing Organic Reactions
1/51
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
acid–base reaction
an acid and a base react, resulting in the formation of the conjugate base of the acid and the conjugate acid of the base; proceeds so long as the reactants are more reactive, or stronger, than the products that they form
Lewis acid
electron acceptor; electrophiles; vacant p-orbitals into which they can accept an electron pair, or are positively polarized atoms
Lewis base
electron donor; nucleophiles; lone pair of electrons that can be donated, and are often anions, carrying a negative charge
coordinate covalent bonds
covalent bonds in which both electrons in the bond came from the same starting atom (the Lewis base)
Brønsted–Lowry acid
a species that can donate a proton (H+)
Brønsted–Lowry base
species that can accept a proton
amphoteric
ability to act as either Brønsted−Lowry acids or bases
ex. water
acid dissociation constant (Ka)
measures the strength of an acid in solution
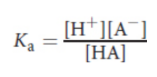
pKa
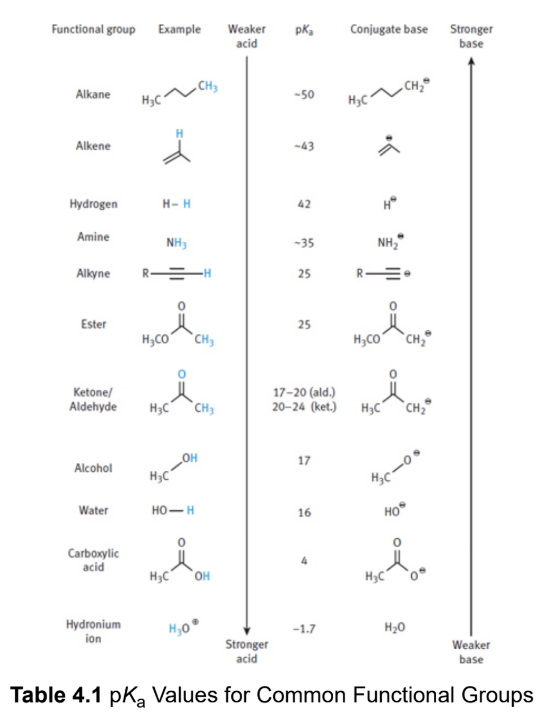
measure of acid strength; smaller numbers are more acidic; acids with a pKa below −2 are considered strong acids
pKa= - log Ka
α-carbon
carbon adjacent to carbonyl group
α-hydrogens
connected to the α-carbon, very acidic since carbonyl-containing carbanions is stabilized by resonance
acidic functional groups
alcohols, aldehydes and ketones (at the α-carbon), carboxylic acids, and most carboxylic acid derivatives
basic functional groups
amines and amides
Nucleophiles
“nucleus-loving” species with either lone pairs or π bonds that can form new bonds to electrophilesnu
carbon, hydrogen, oxygen, or nitrogen (CHON) with a minus sign or lone pair
nucleophilicity
based on relative rates of reaction with a common electrophile; kinetic property
increases with negative charge
decreases with electonegativity
decreases with steric hinderance
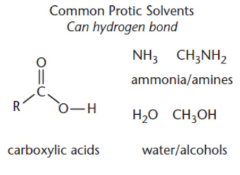
protic solvents hinder
polar protic solvent
hinder nucleophilicity by protonating the nucleophile or through hydrogen bonding
I- → Br- → Cl- → F-
polar aprotic solvents,
F- → Cl- → Br- → I-
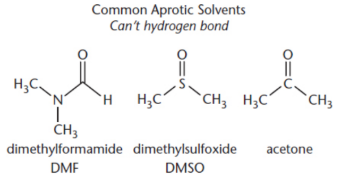
nonpolar solvents
can’t use nucleophile–electrophile reactions because reactants are polar and wouldn’t dissolve
strong nucleophiles
HO– , RO– , CN–
fair nucleophiles
N3-, NH3, RCO2-
weak nucleophiles
H2O, ROH, RCOOH
nucleophilic functional groups
amine
Electrophiles
“electron-loving” species with a positive charge or positively polarized atom that accepts an electron pair when forming new bonds
electrophilicity
based on relative rates of reaction with a common nucleophile; kinetic property
increases with positive charge
improved by good leaving groups
Leaving groups
molecular fragments that retain the electrons after heterolysis
must be able to stabilise the electrons - weak bases, resonance, inductive effects
ex. conjugate bases of strong acids
Heterolytic reactions
the opposite of coordinate covalent bond formation: a bond is broken and both electrons are given to one of the two products
Nucleophilic substitution reactions
a nucleophile forms a bond with a substrate carbon and a leaving group leaves
Unimolecular nucleophilic substitution (SN1) reactions
rate-limiting step in which the leaving group leaves, generating a positively charged carbocation; the nucleophile then attacks the carbocation, resulting in the substitution product
rate = k[R−L], where R−L is an alkyl group containing a leaving group (first order)
racemic mixture.
![<p>rate-limiting step in which the leaving group leaves, generating a positively charged carbocation; the nucleophile then attacks the carbocation, resulting in the substitution product</p><p>rate = k[R−L], where R−L is an alkyl group containing a leaving group (first order)</p><p>racemic mixture.</p>](https://knowt-user-attachments.s3.amazonaws.com/8c5205cb-a67b-4f5b-9e21-5a0374d1d274.png)
Bimolecular nucleophilic substitution (SN2) reactions
the nucleophile attacks the compound at the same time as the leaving group leaves
rate = k[Nu:][R−L] (second order)
![<p>the nucleophile attacks the compound at the same time as the leaving group leaves</p><p>rate = k[Nu:][R−L] (second order)</p>](https://knowt-user-attachments.s3.amazonaws.com/fe6f0da1-9c57-4819-84fc-32753bf7f451.png)
concerted reaction
takes one step
backside attack
nucleophile attacks electrophile from opposite direction of leaving group, leading to stereotopic Walden inversion
stereospecific reaction
the configuration of the reactant determines the configuration of the product due to the reaction mechanism
oxidation–reduction (redox) reactions
the oxidation states of the reactants change
Oxidation state
indicator of the hypothetical charge that an atom would have if all bonds were completely ionic
Hydrogen = +1
Oxygen = -2
ion = charge
relative oxidation of functional groups
most oxidation
carbon dioxide
carboxylic acids, anhydrides, esters, amides
aldehydes, ketones, imines
alcohols, alkyl halides, amines
alkanes
Oxidation
increase in oxidation state; loss of electrons; increasing the number of bonds to oxygen or other heteroatoms
Reduction
decrease in oxidation state; gain in electrons; increasing the number of bonds to hydrogen
oxidizing agent
element or compound that accepts an electron from another species; gets reduced
have a high affinity for electrons (such as O2, O3, and Cl2) or unusually high oxidation states (Mn7+ in permanganate and Cr6+ in chromate).
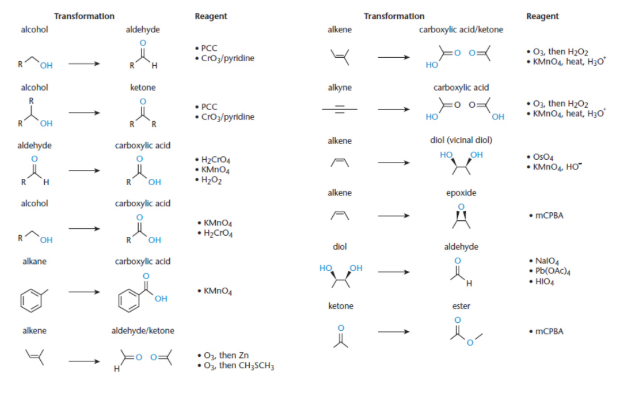
Oxidation of primary alcohols
one level to become aldehydes using specific reagents such as pyridinium chlorochromate (PCC)
further oxidized to form carboxylic acids with strong oxidising agents chromium trioxide (CrO3) or sodium or potassium dichromate (Na2Cr2O7 or K2Cr2O7)
Oxidation of primary alcohols
turn into ketones
reducing agents
sodium, magnesium, aluminum, and zinc - low electronegativities and ionization energies
transition metals can often take on many different oxidation states.
Metal hydrides - NaH, CaH2, LiAlH4, and NaBH4
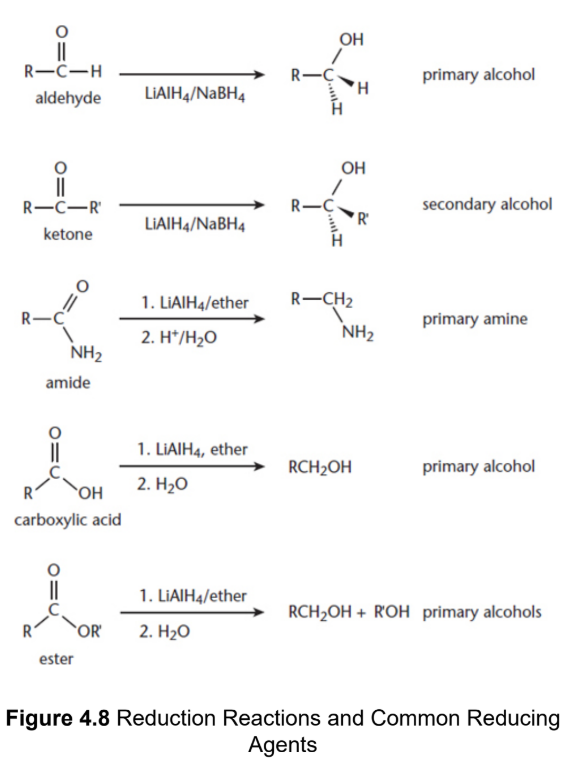
Reduction of aldehydes and ketones
primary and secondary alochols, respectively
Reduction of amides
turns into amines using LiAlH4
Reduction of carboxylic acids
turn to primary alcohols using LiAlH4
Reduction of esters
turn into a pair of primary alcohols LiAlH4
chemoselectivity
the preferential reaction of one functional group in the presence of other functional groups
redox agent chemoselectivity
highest priority group, most oxidized carbon
ex. carboxylic acid > aldehyde > ketone > alcohol/amine
nucleophile-electrophile chemoselectivity
highest priority group; more electronegative groups around
ex. carboxylic acid > aldehyde > ketone > alcohol/amine
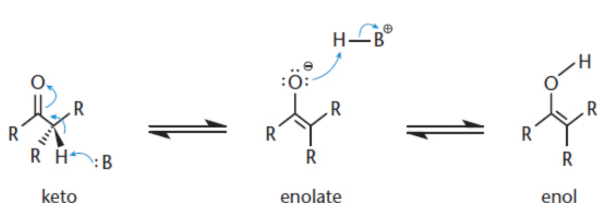
carbon of a carbonyl
positive polarity due to the electronegativity of the oxygen
the α-hydrogens are much more acidic than in a regular C−H bond due to the resonance stabilization of the enol form
enolate then becomes a strong nucleophile, and alkylation can result if good electrophiles are available
substrate carbon in substitution reactions
SN1 reactions - tertiary > secondary > primary - carbocation stability
SN2 reactions - methyl > primary carbons > secondary x tertiary - steric hindrance
Steric hindrance/protection
the prevention of reactions at a particular location within a molecule due to the size of substituent groups; bulky groups make it impossible for the nucleophile to reach the most reactive electrophile, making the nucleophile more likely to attack another region
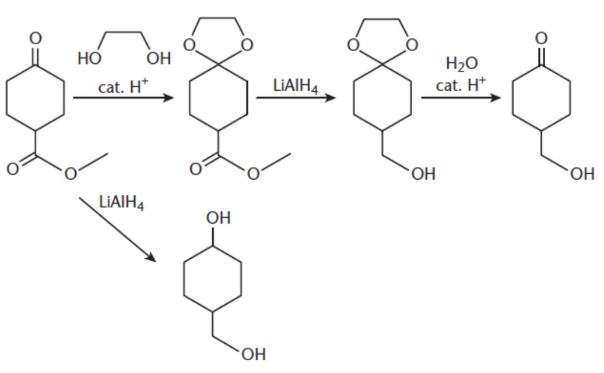
protecting group
group causing steric hindrance
ex. aldehyde or ketone converted to a nonreactive acetal or ketal