Unit 2 Topic 7b: VSEPR Theory & Molecular Geometry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
VSEPR Theory
The Valence Shell Electron Pair Repulsion Theory helps us explain why molecules take the shapes they do
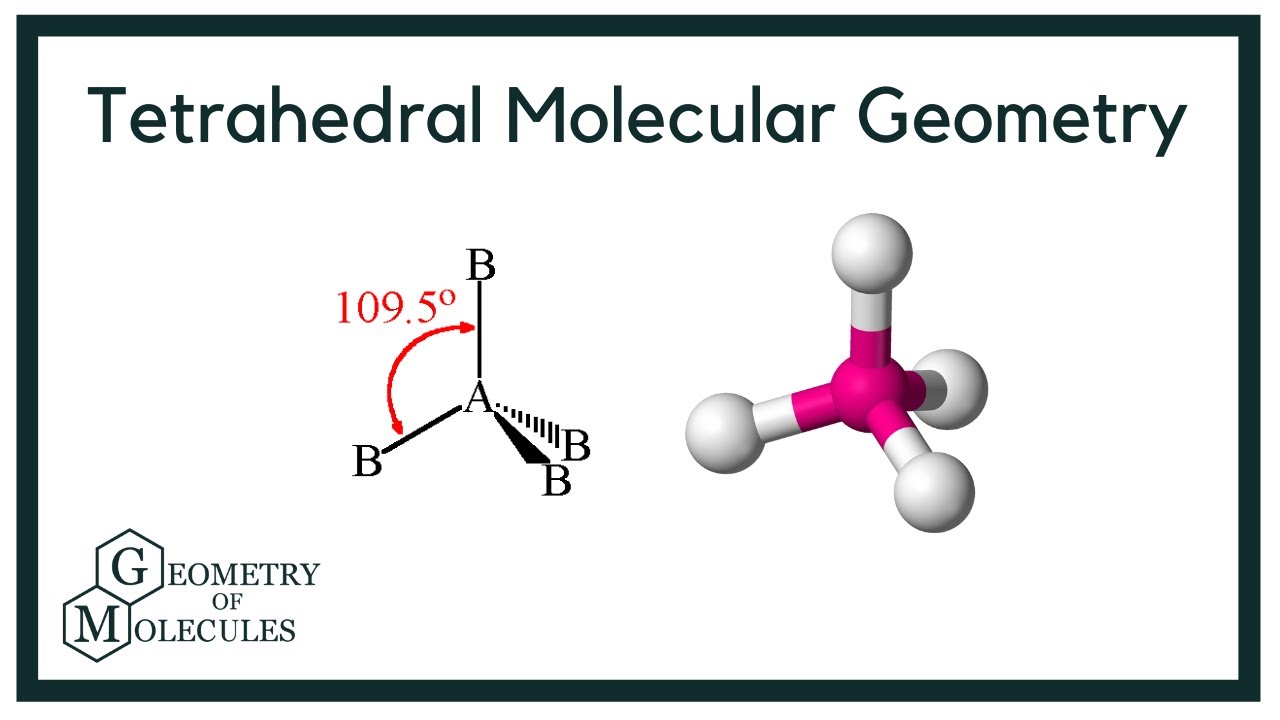
Tetrahedral
4 sigma bonds
0 unshared pairs
bond angle 109.5
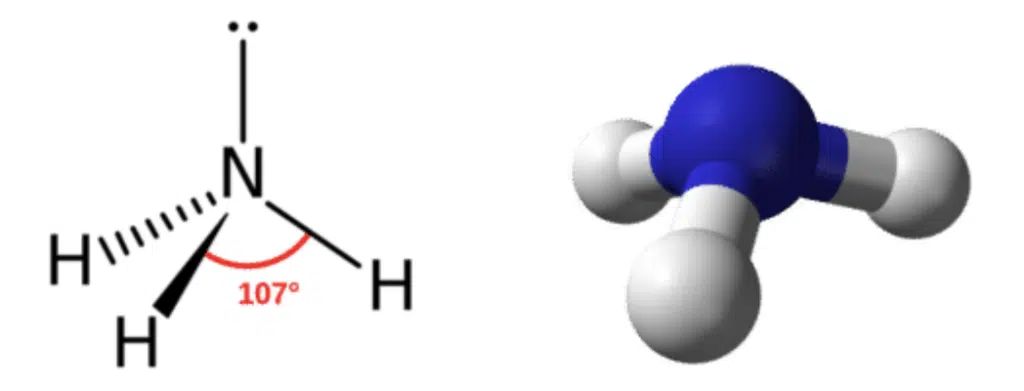
Trigonal pyramidal
3 sigma bonds
1 unshared pair
Bond angle 107
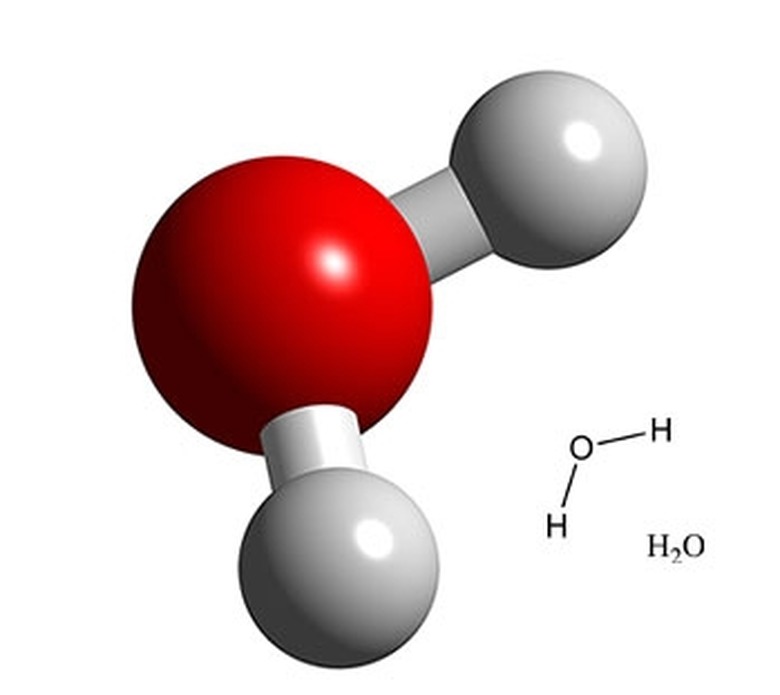
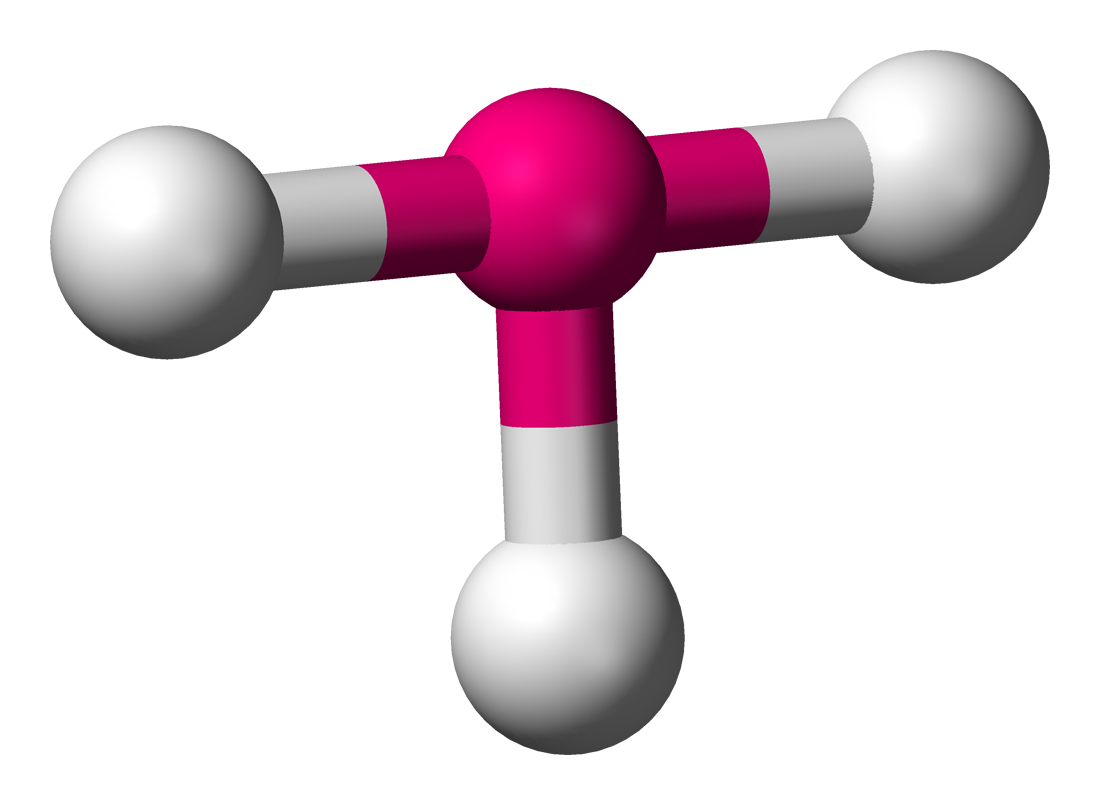
Bent
2 sigma bonds
2 unshared pairs
bond angle 105
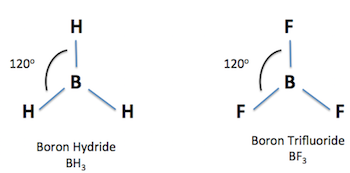
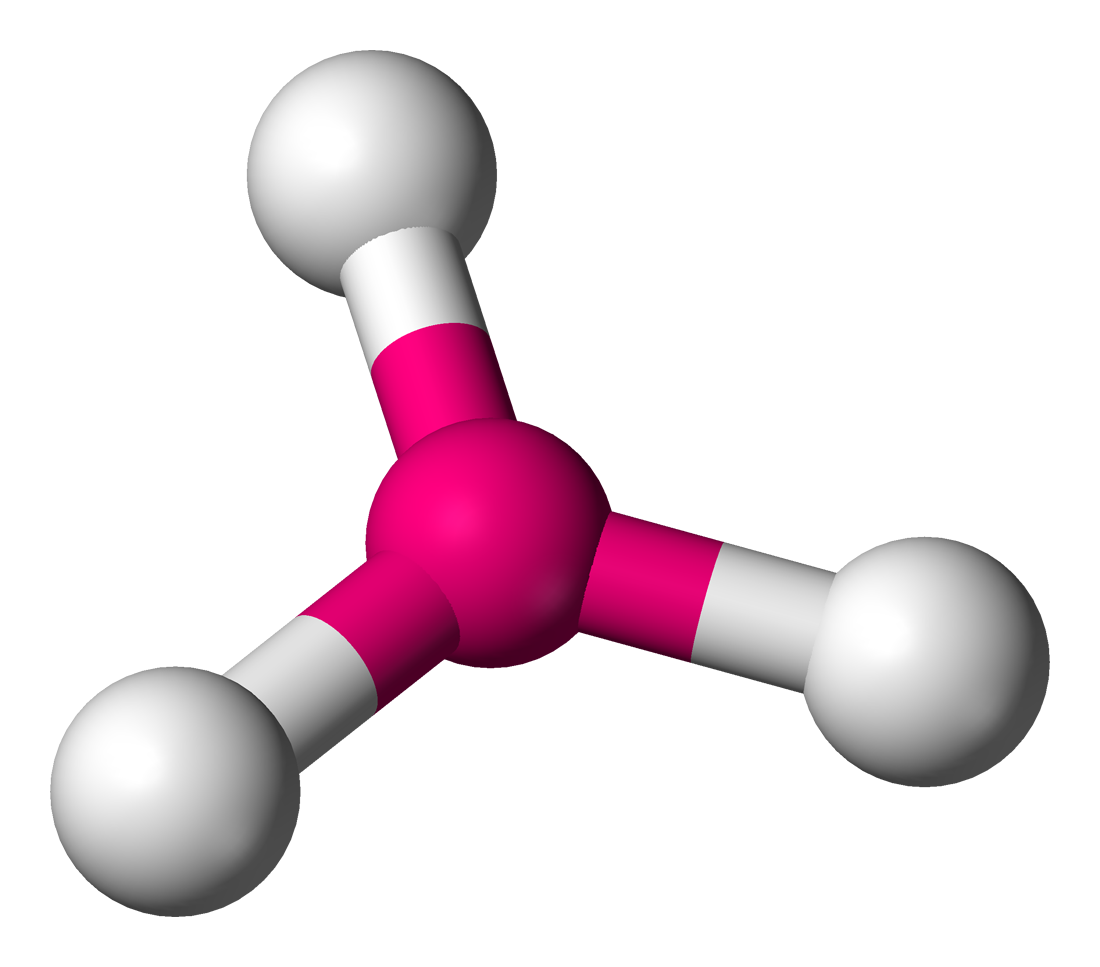
Trigonal Planar
3 sigma bonds
0 unshared pairs
bond angle 120
Angular
2 sigma bonds
1 unshared
bond angle 117
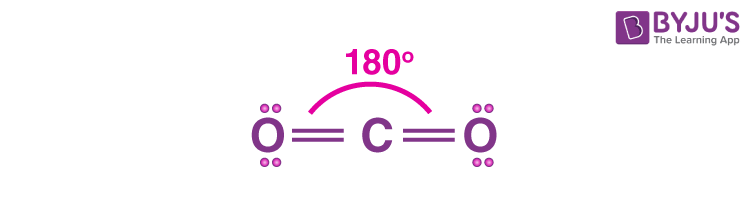
Linear
2 sigma bonds
0 unshared pair
bond angle 180
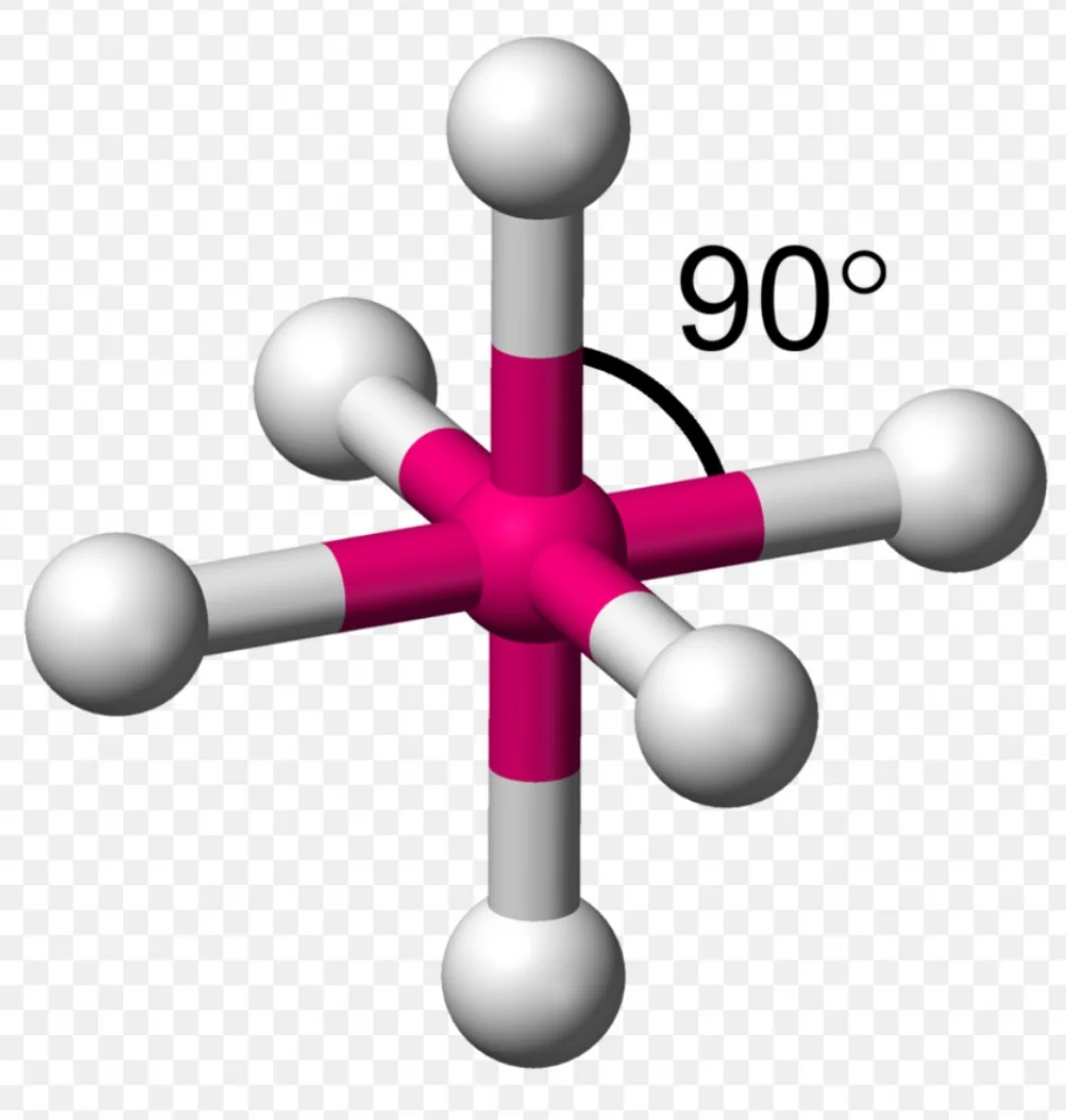
Octahedral
6 sigma bonds
0 unshared bonds
bond angle 90
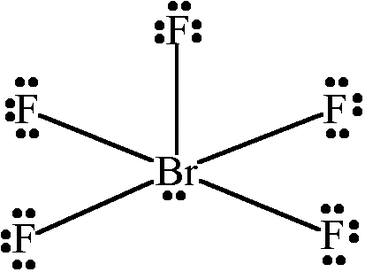
Square pyramidal
5 sigma bonds
1 unshared bonds
bond angle 90
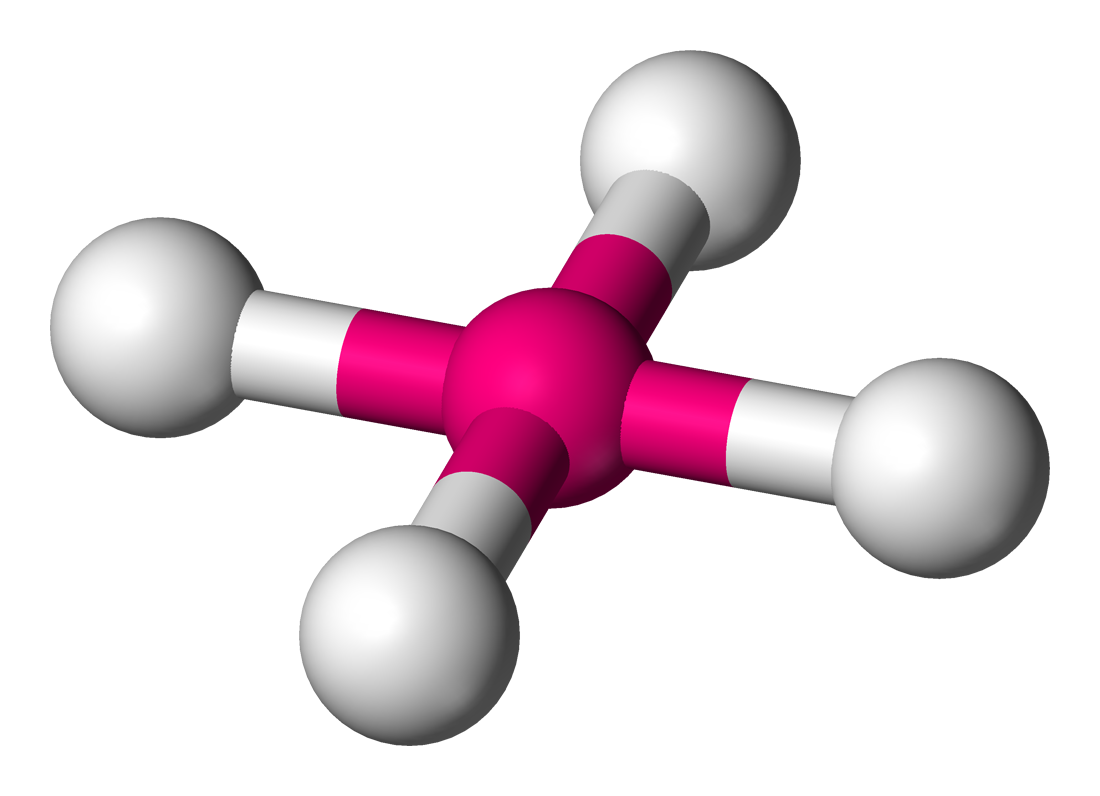
Square Planar
4 sigma bonds
2 unshared bonds
bond angle 90
T-Shaped
3 sigma bonds
3 unshared bonds
bond angle 90 and 180
Trigonal Bipyramidal
5 sigma bonds
0 unshared bonds
bond angles 90 and 120

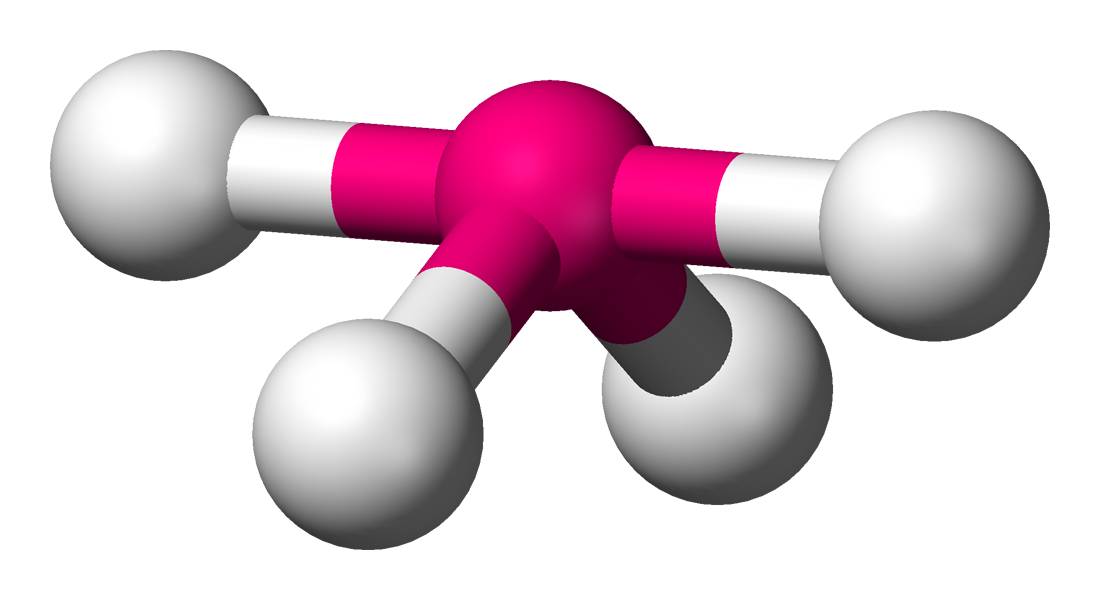
Seesaw
4 sigma bonds
1 unshared bonds
bond angles 87 and 117
Trigonal planar
3 sigma bonds
2 unshared bonds
bond angle 120
SNAP
Symmetrical Nonpolar- Balanced, bond dipoles cancel each other out
Asymmetrical Polar - has lone pairs on central atom, different electronegativity between bonds (lopsidedness)