Microbio Exam 3: Immunology
1/208
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
209 Terms
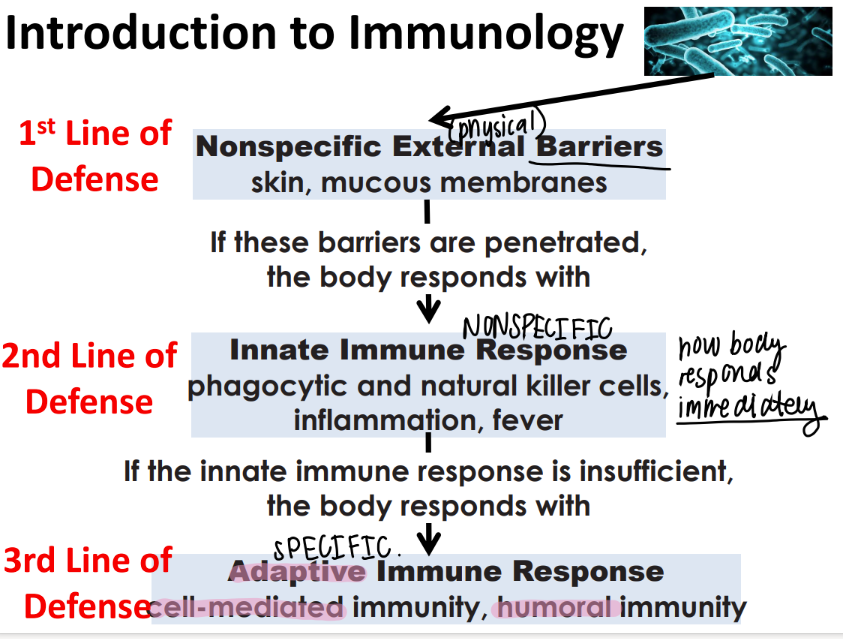
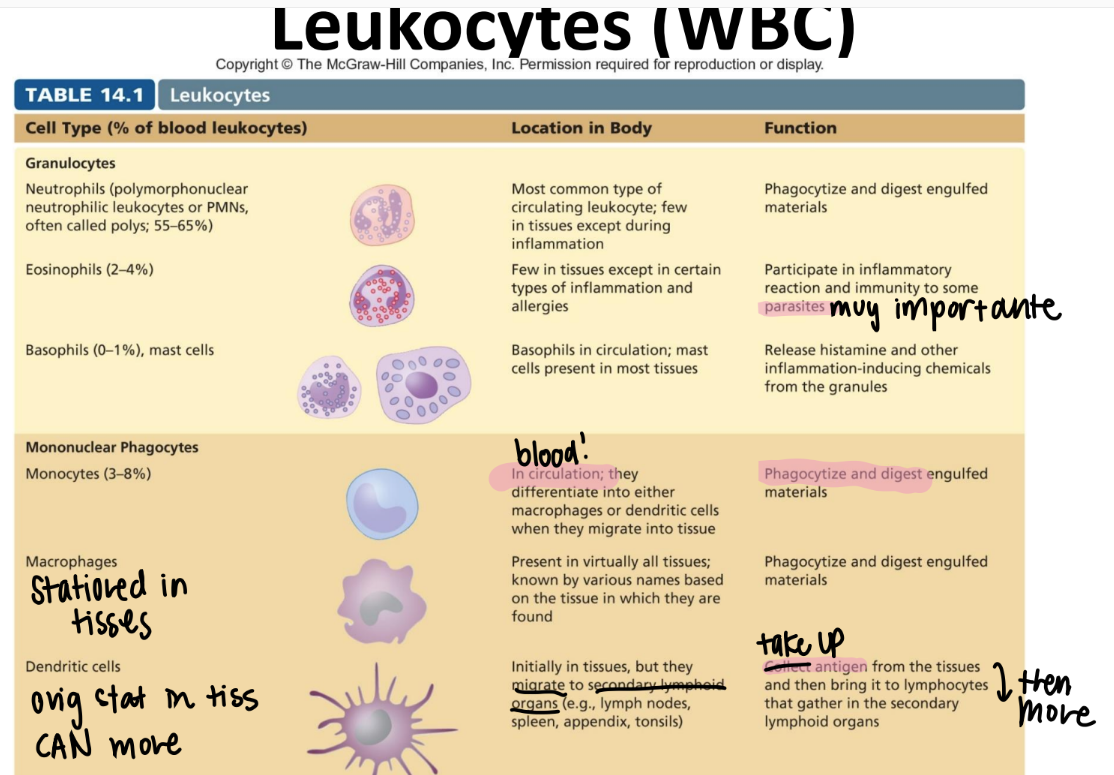
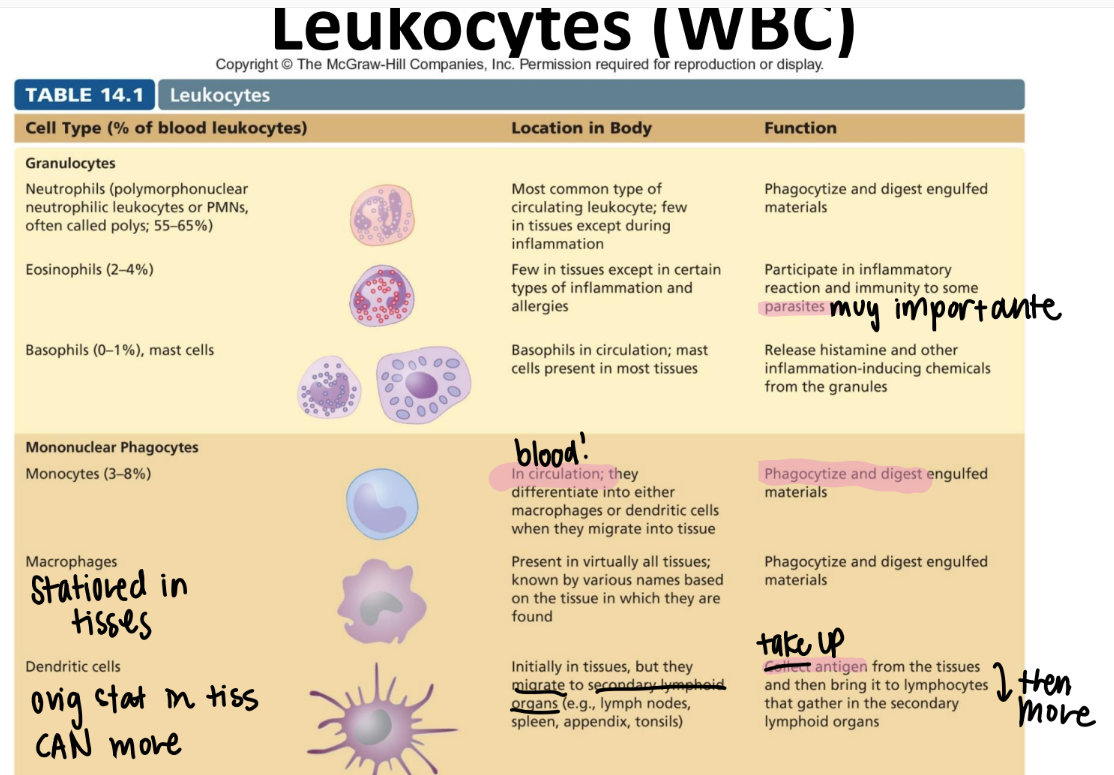
what are the 3 lines of defense in immunology?
nonSPECIFIC external barriers (skin)
nonspecific INNATE immune res (killer cells, inflammation, fever)
adaptive imm res (cell mediated, humoral imm)
tf is innate immunity?
pros and cons?
BROAD recog of PATHOGENS via PAMPs.
pros: born w/ it, fast
cons: no mem

tf is adaptive imm: WHO does the recognizing, and WHAT do they recognize?
humoral (B cells) / cellular (T cells) pre-existing CLONES recog antigens on pathogens.
→ B cells make antibodies
→ T cells present antigens
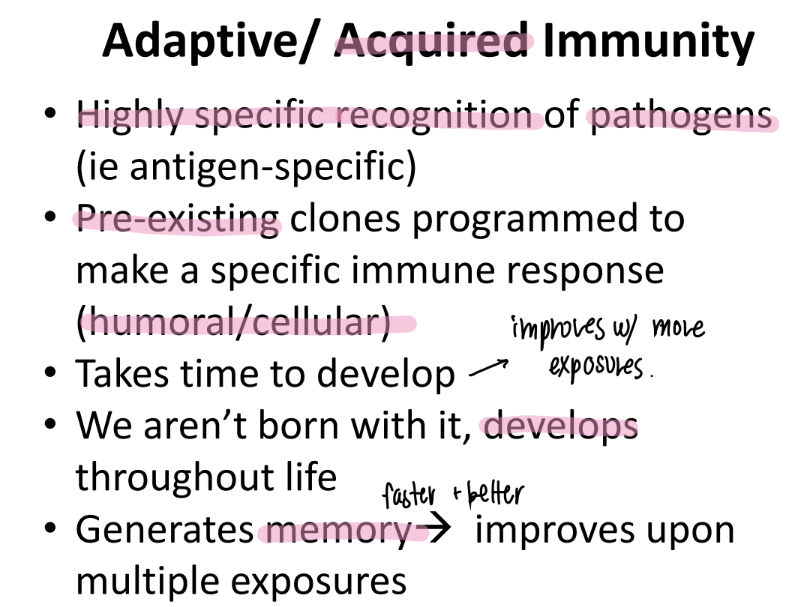
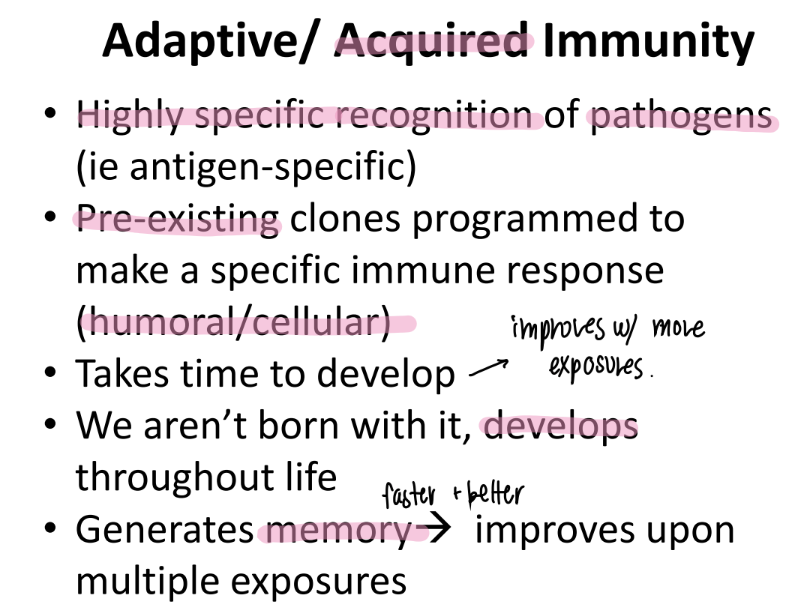
what are the pros and cons of adaptive imm?
pros: develops throout life = takes time
cons: memory, highly specific → improves w/ each exposure!
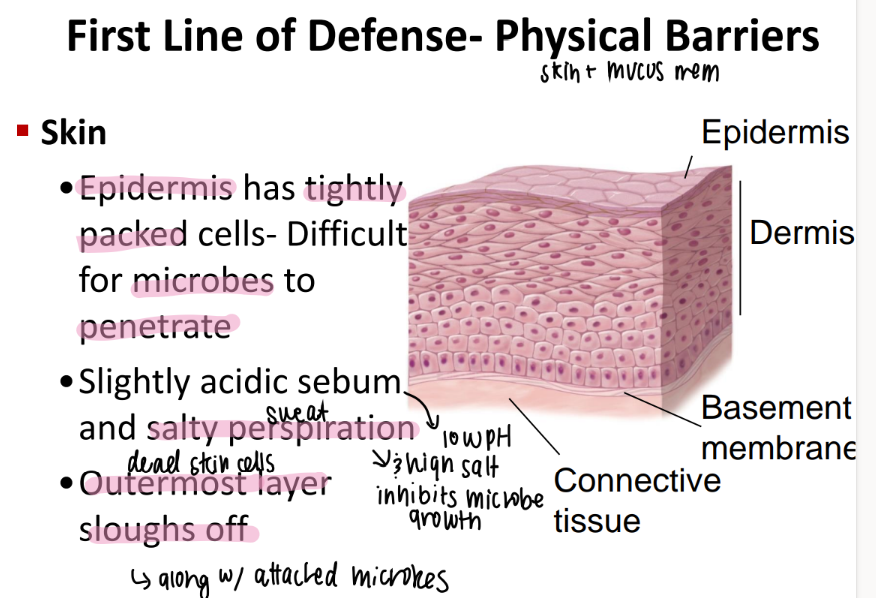
what properties of the skin make it a great external barrier to bacteria?
tightly packed epidermis = hard to puncture!
acidic sebum + salty sweat = inhibits growth
outermost skin cells die off!
what are the 2 physical barriers that make up the “nonspecific external barrier”?
skin + mucous membranes
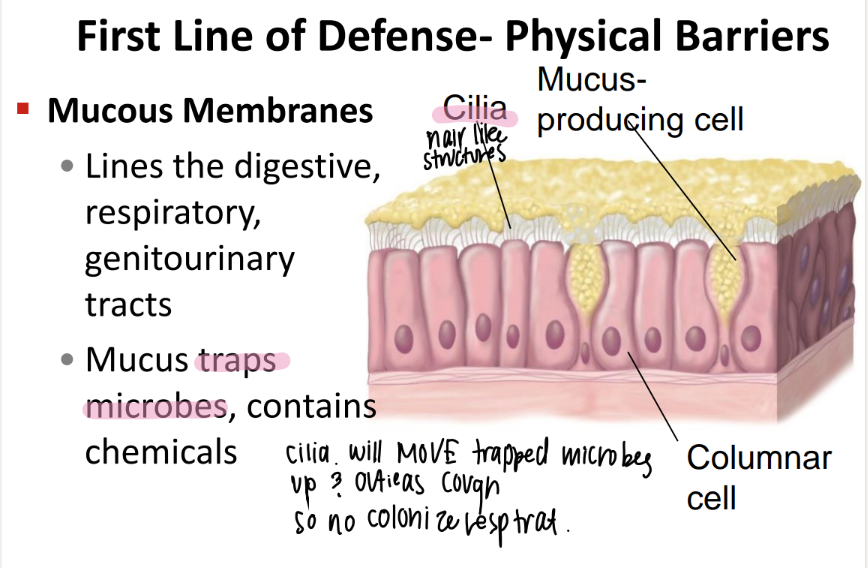
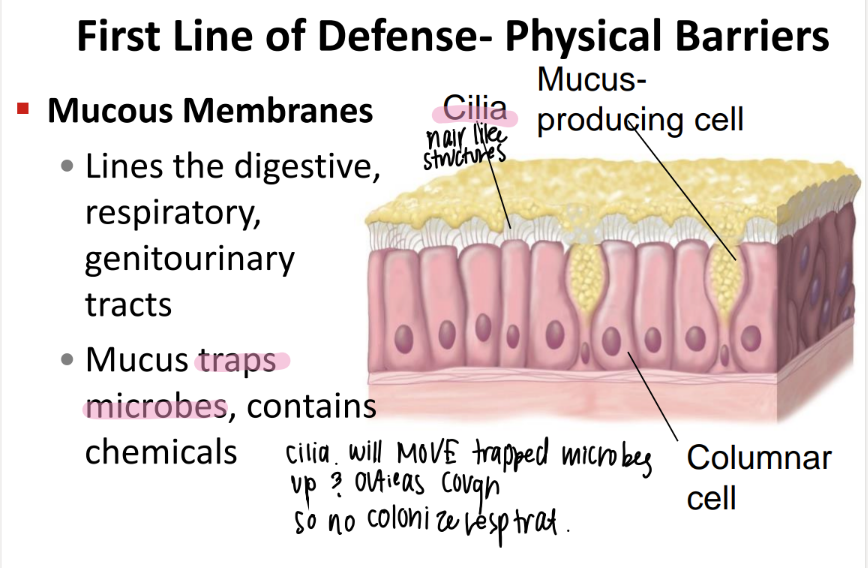
what’s the purpose of the mucous membranes?
where r they?
traps microbes + contains chemicals.
digestive, respiratory, genitourinary tracts
what physical movement protects the respiratory tract from microbes?
mucocilliary escalator
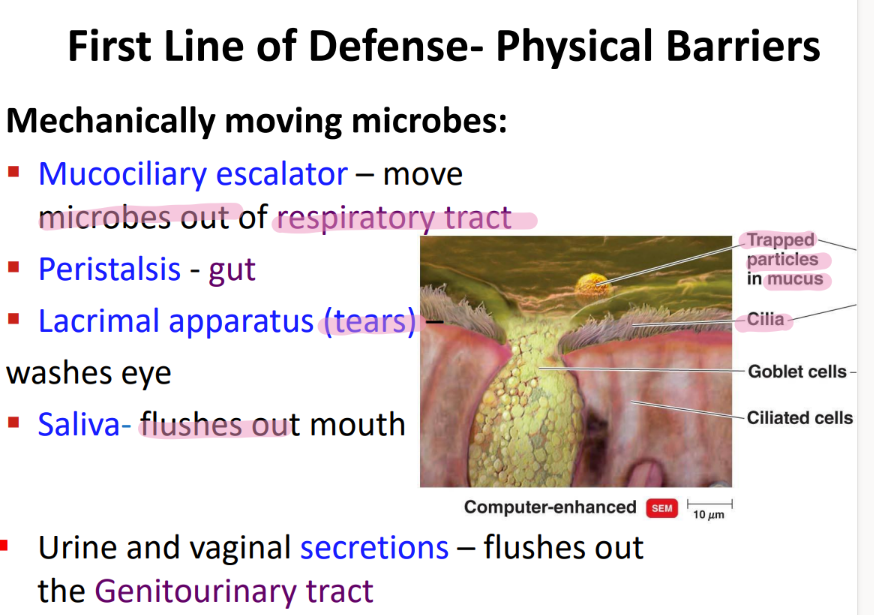
what physical movement protects the gut from microbes?
PERISTALSIS.
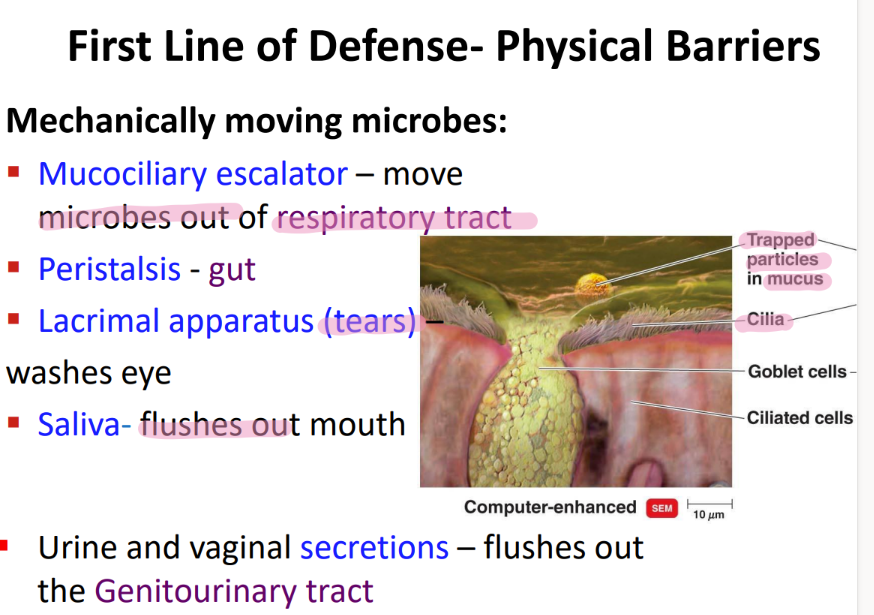
what physical movement protects the eye from microbes?
tears / LACRIMAL apparatus
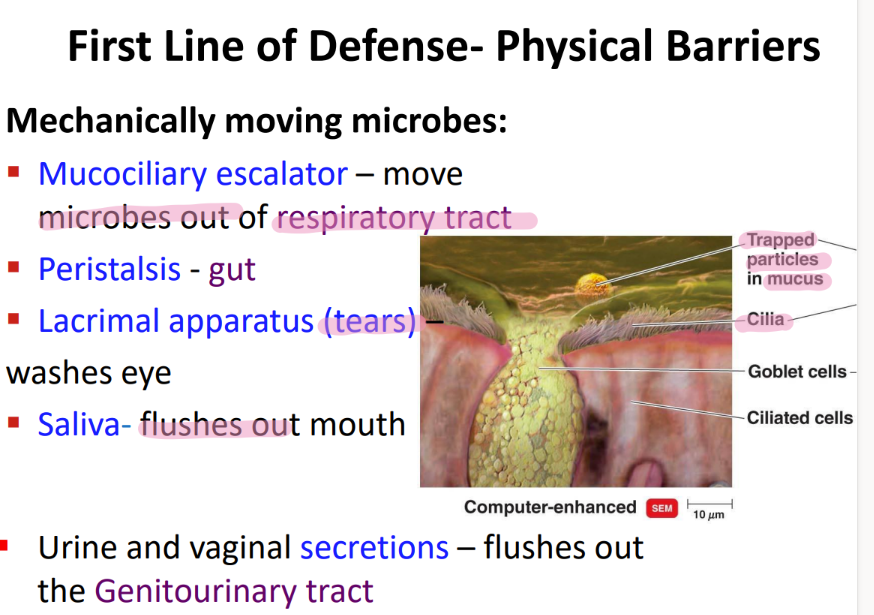
what physical movement protects the mouth from microbe?
saliva!
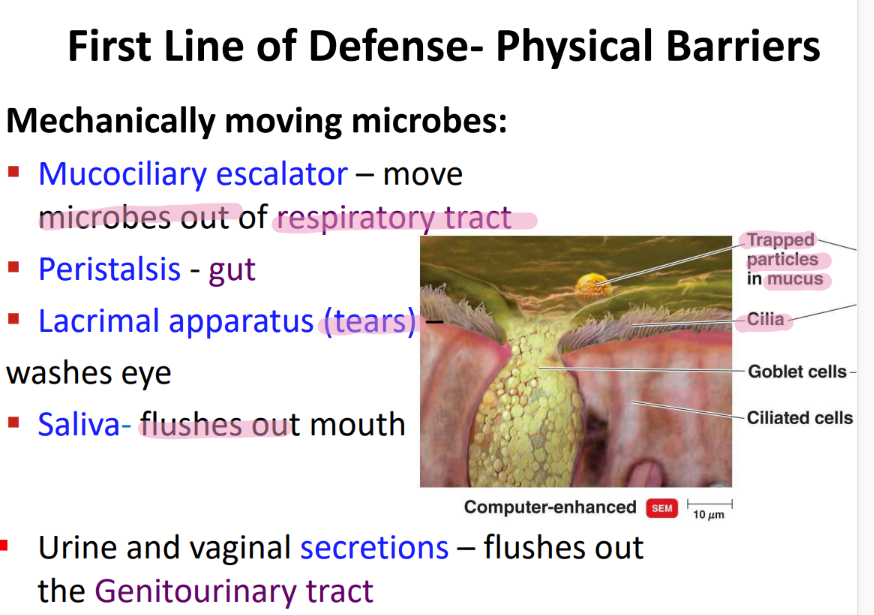
what physical movement protects the genitourinary tract?
urine + vaginal SECRETIONS.
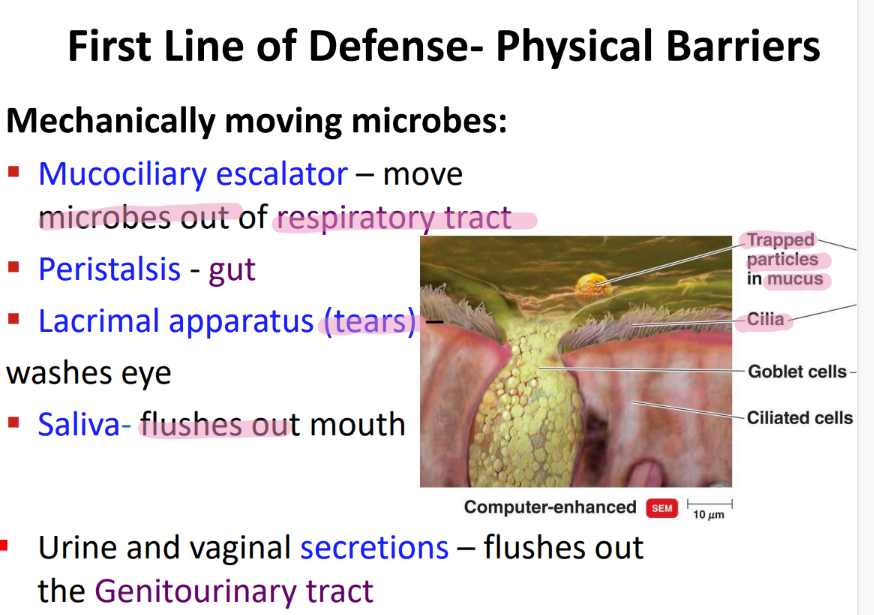
lysozyme is found where and does what?
found in tears / saliva / misc secretions
CLEAVES bonds btw NAM + NAG!
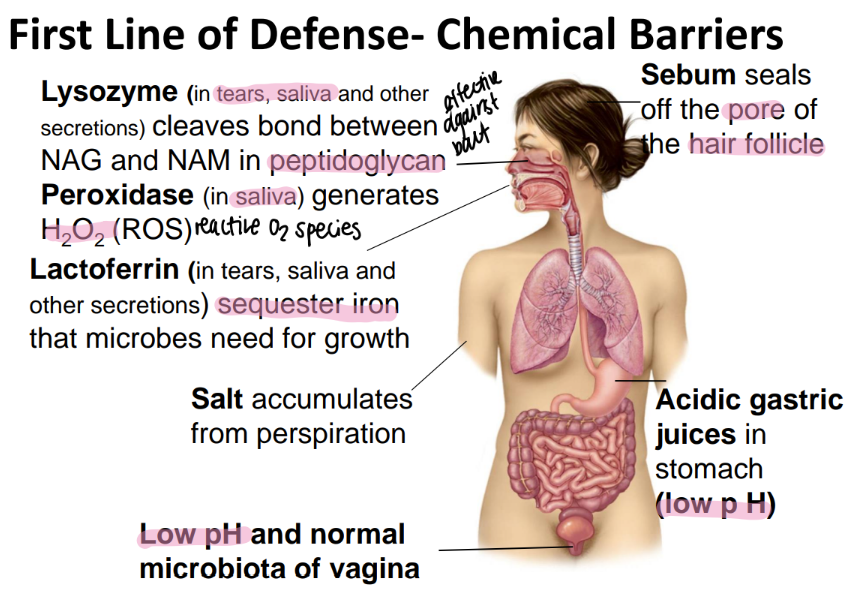
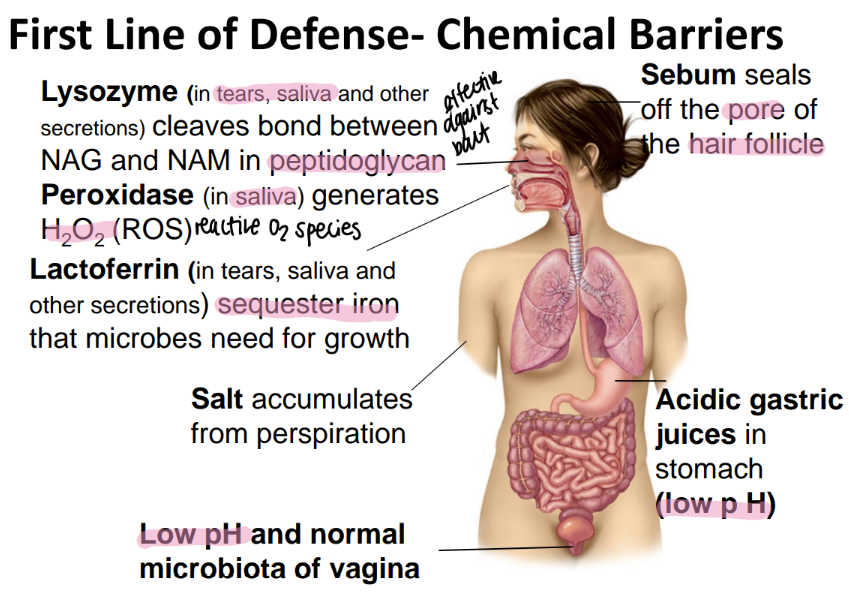
perioxidase is found where and does what?
generates HYDROGEN peroxide which is a REACTIVE oxygen species (ROS)
found in SALIVA
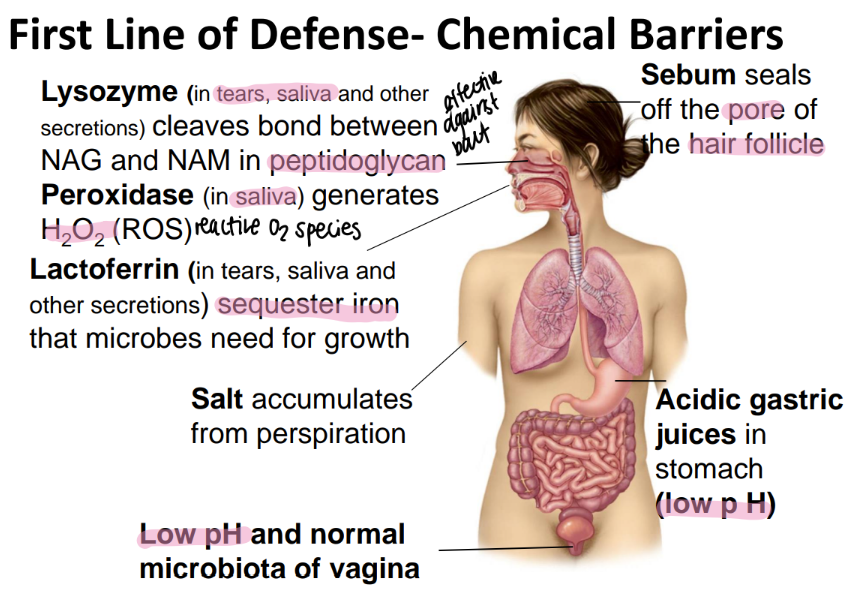
lactoferrin is found where and does what?
ferrin= iron!!!
found in tears/ saliva/ secretions
binds to free iron so microbes can’t have it
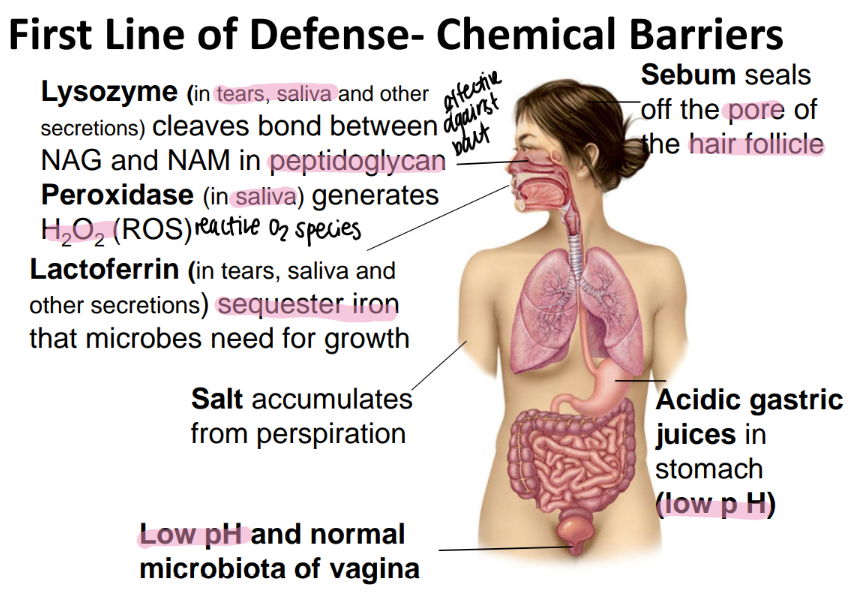
salt is found where and does what?
sweat
dries out microbes
what chemical barriers protect the vagina?
low pH (acidic)
microbiata
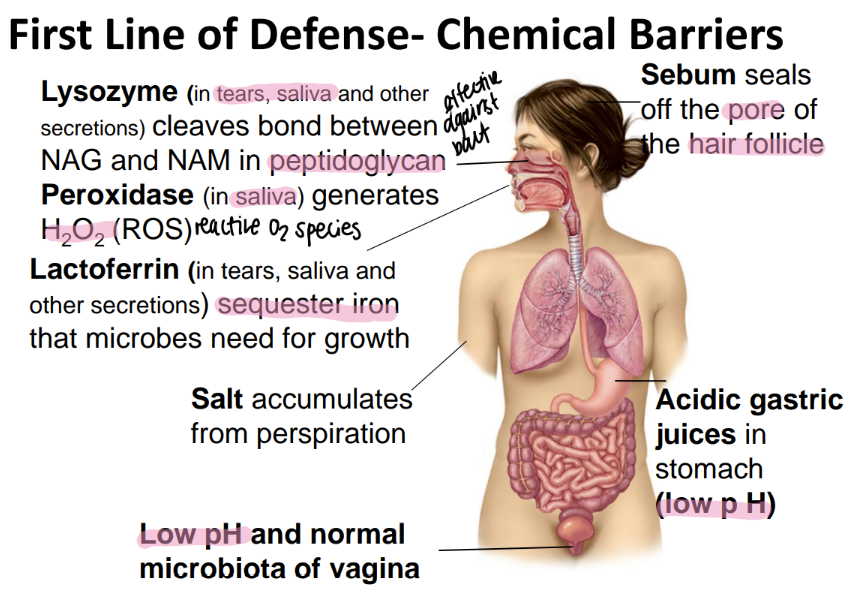
sebum is found where and does what?
seals off the HAIR follicle
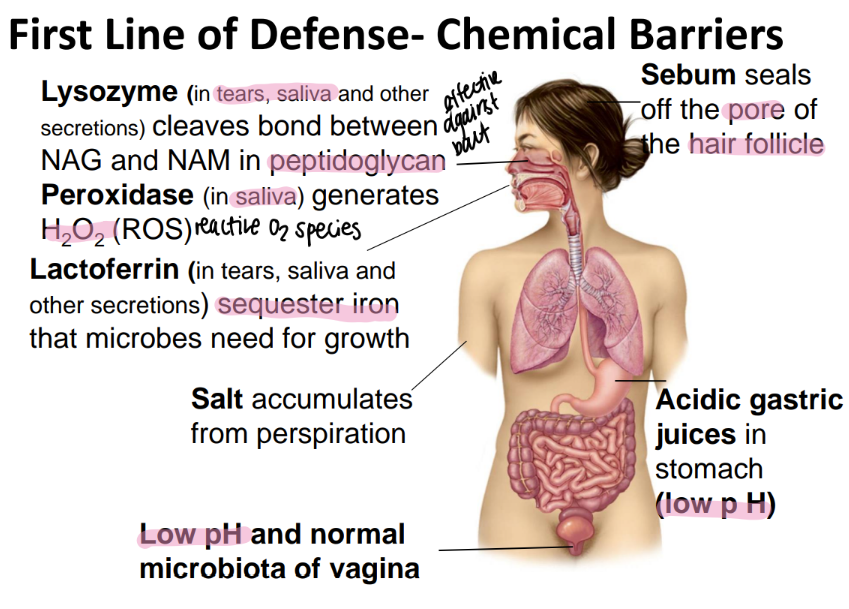
how does the stomach chemically protect itself from microbes?
low pH via acidic gastric juices
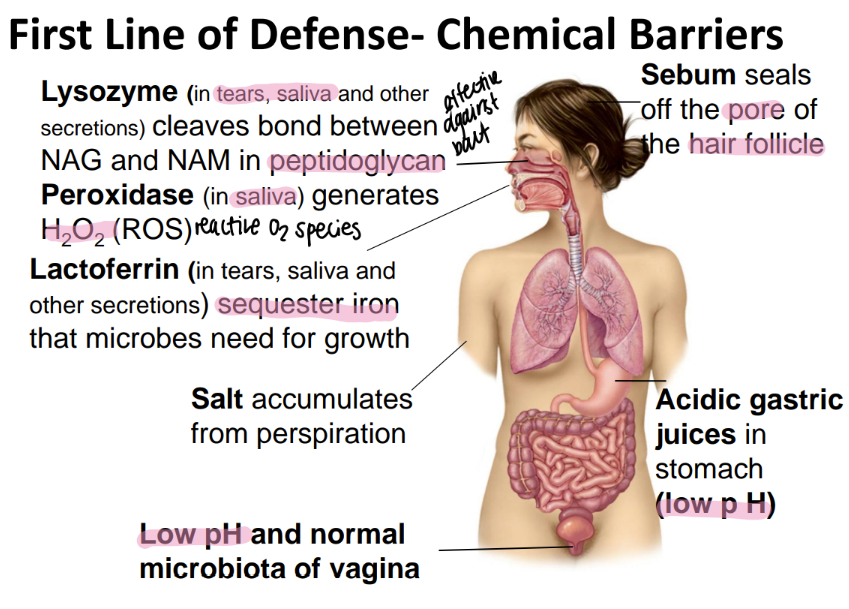
what “cellular barrier” acts as a first line of defense against microbes?
how does it work?
MICROBIATA!
take up space= inhib growth
secrete chemicals to kill

what cells serve as the “bridge” btw the innate and adaptive?
why?
macrophages + dendritic cells
present ANTIGENS to the adaptive imm sys
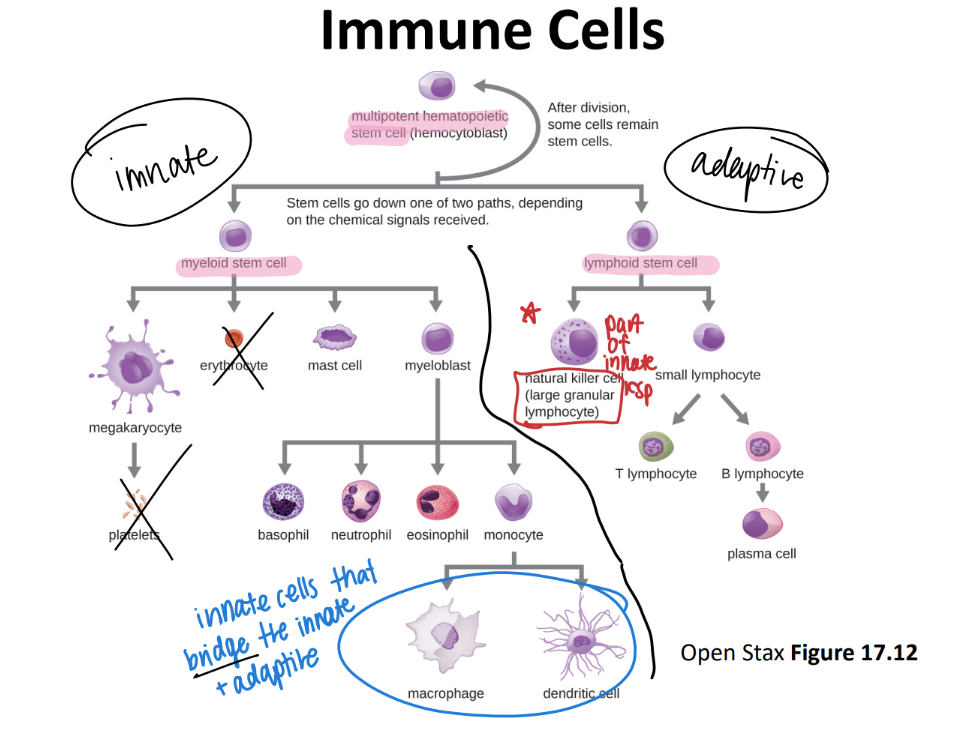
where do all immune cells originate from?
multipoetic stem cell!
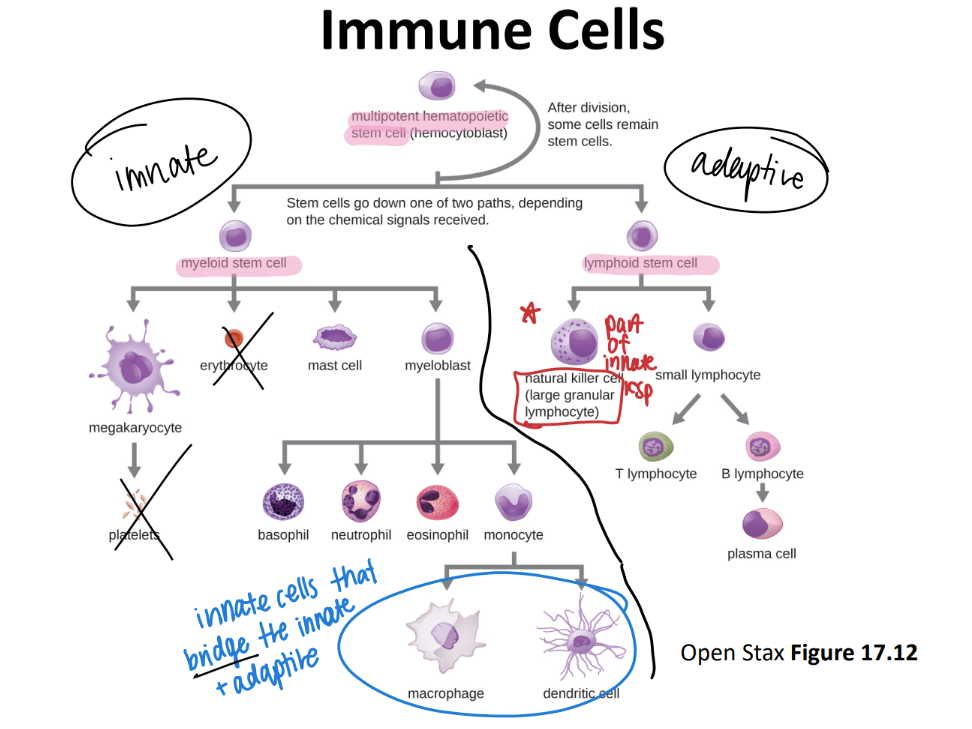
immune cell pathway: what are most innate imm cells made from? hb adaptive?
inn: myeloid stem cell
adaptive: lymphoid stem cell
innate: Never Let Monkeys Eat Bananas (neutrophils, mast cells, eosinophils, basophils)
tf is the difference between a leukocyte and a lymphocyte?
leukocyte = WBC
lymphocyte = a SPECIFIC WBC swimming in the lymph that recognizes ANTIGENS.

monocytes!!
purpose?
where r they?
what are they classified as?
DIFFERENTIATE into macrophages/ dendritic cells when they move from the blood INTO the tissue.
eat and digest
mononuclear phagocyte! (leukocyte since it’s a WBC)
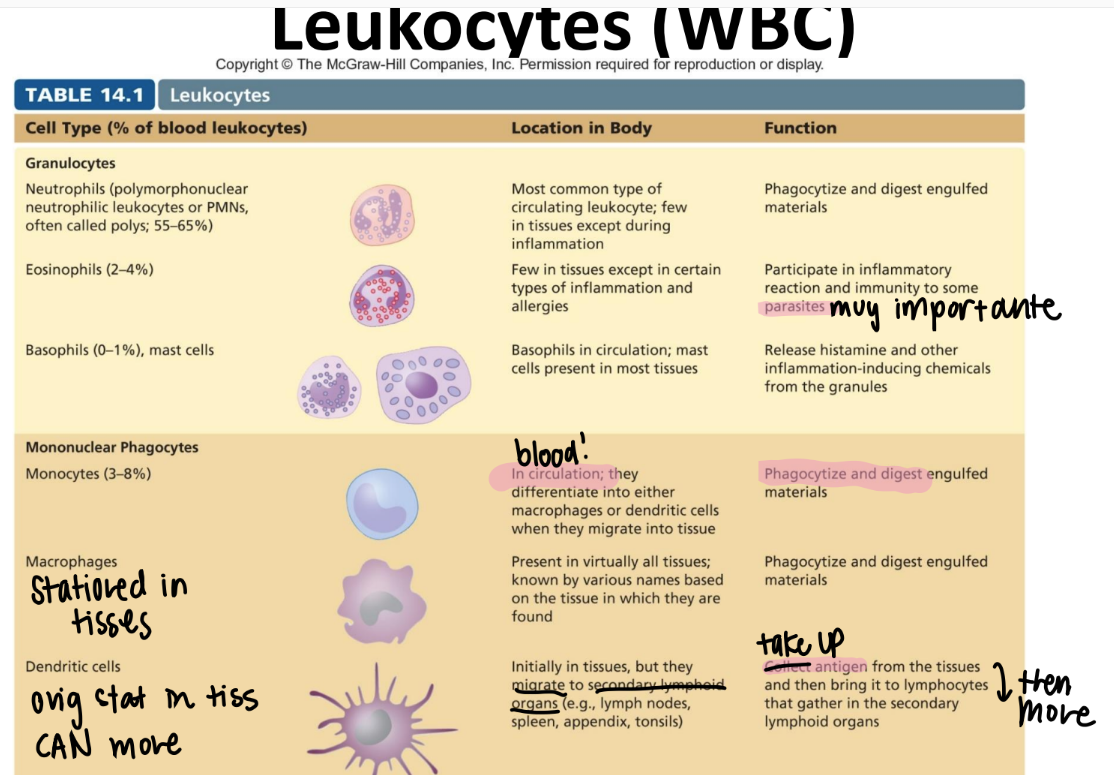
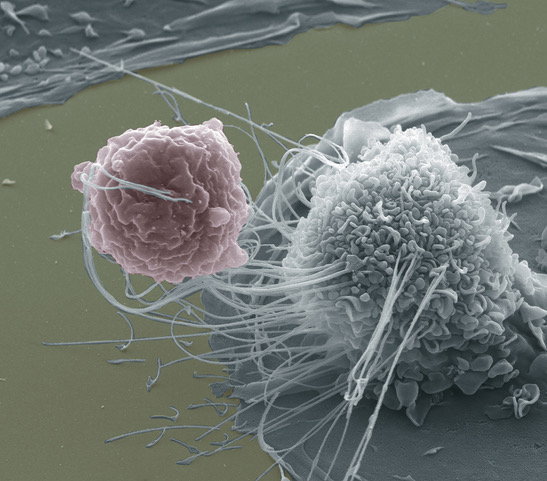
what’s the function of the macrophage?
are they in tissues or circulating?
where r they?
what are they classified as?
stationed IN TISSUES. NOT circulation.
eat + digest
“mononuclear phagocyte” leukocyte
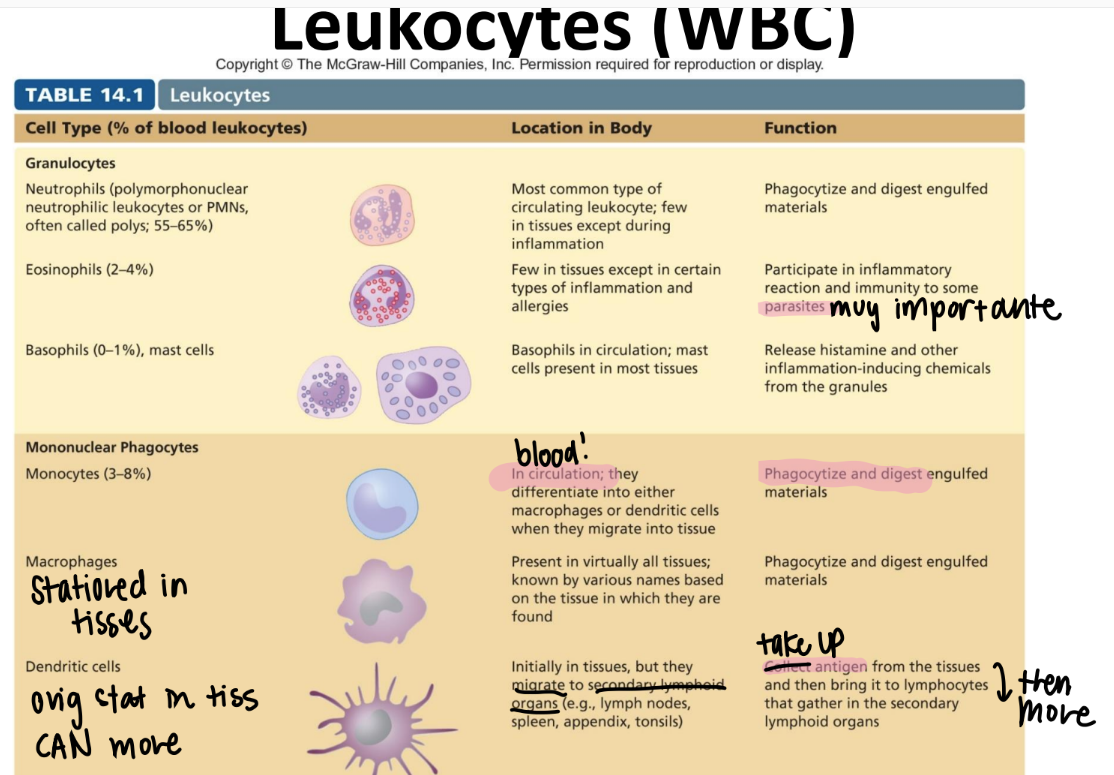
what’s the function of the dendritic cell?
what’s it classified as?
relay antigens (from tissues) to lymphocytes in 2ndary lymph organs (ie appendix, spleen, lymph nodes)
MONOnuclear phagocyte.
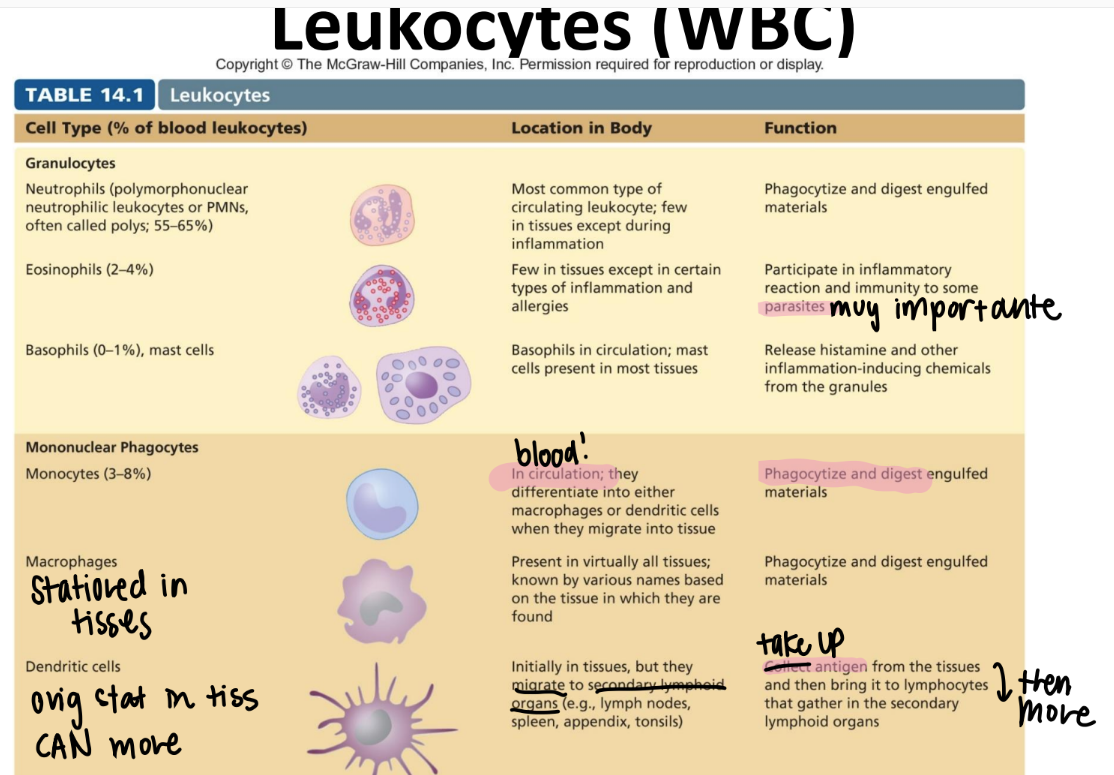
what’s the function of the neutrophil? (aka?)
circulating or in tissue?
what are they classified as?
PMN!
circulating WBC; phagocytize + activate inflammation
GRANULOCYTES (leukocyte)
what’s the difference between the neutrophil and NK (natural killer) cell? both are innate!
neutrophil: leukocyte = phagocytize microorg’s like bact
NK: granulocyte = PERFORATE: perforins, granzymes, cytokines on viral / cancer infected cells bc faulty MHC I rec.
what’s the function of the mast cell? what’s it aka?
is it circulating or stationed?
BASOPHILS (in blood), then becomes mast cells in tissues
release HISTAMINES + other chem’s
what are secondary lymph organs?
lymph node, appendix, spleen, tonsils
what’s the goal of inflammation, and how does it work (broadly)?
CONTAIN the pathogens, THEN move immune cells + inflammatory proteins to the SITE of infection.
(CDR: contain, destroy, remove)
what happens if acute inflammation fails to kill the pathogen?
CHRONIC inflammation (contain the infection)
what happens if the CHRONIC inflammation response fails?
CONTAIN the infection UNTIL the adaptive imm res FULLY develops

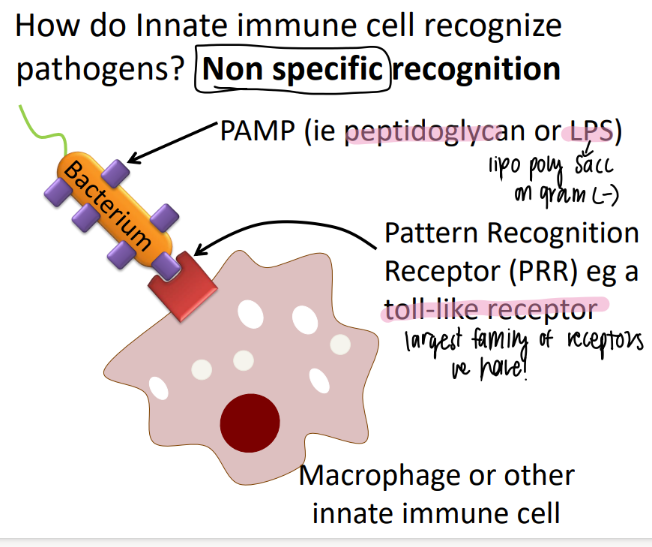
WTF are “pamps”?
pathogen-associated molecular patterns
structures COMMON to non-humans (ie bacteria, viruses)
how are PAMPS relevant to the innate immune sys?
innate may be NON specific, but they still identify non-human bad guys.
what are some examples of PAMPs?
how does the imm sys use them to our advantage?
peptidoglycan, LPS
toll-like / PRR recog the PAMPs
(pattern recog receptor)
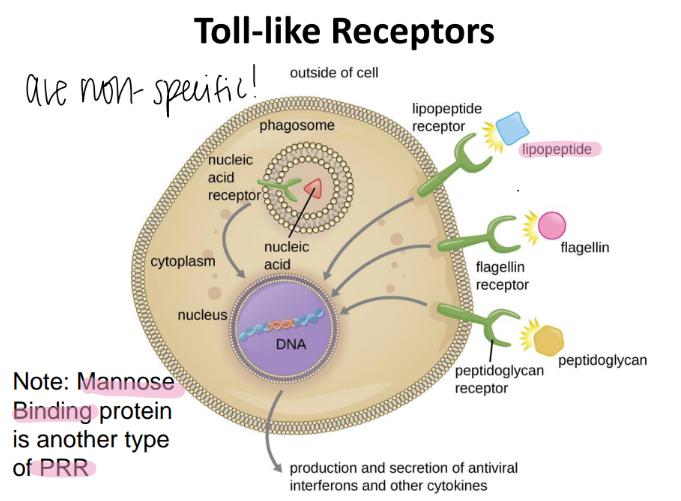
mannose binding protein is an example of what?
a PRR!
(pattern recognition receptor)
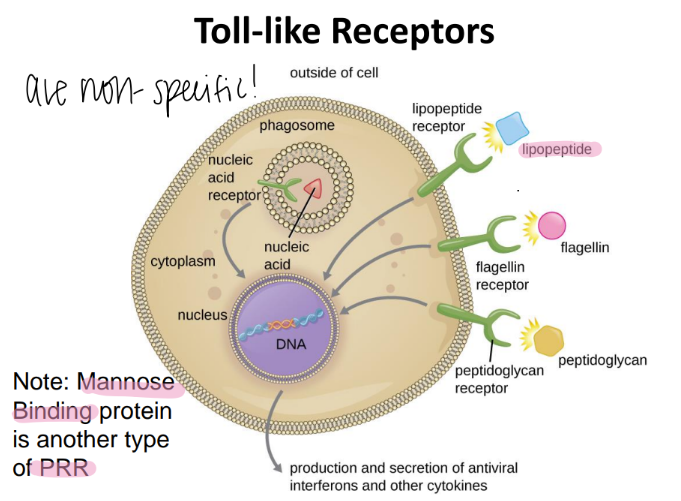
toll like receptors are also called what?
are they specific or not?
NON specific
aka PRR (pattern recognition receptors)
wtf is an opsonin?
a soluable HOST protein
on the OUTER surface of the pathogen
so the imm cell can PHAGOCYTOZE it more easily!
(aka- we slap a big red flag on the pathogen)
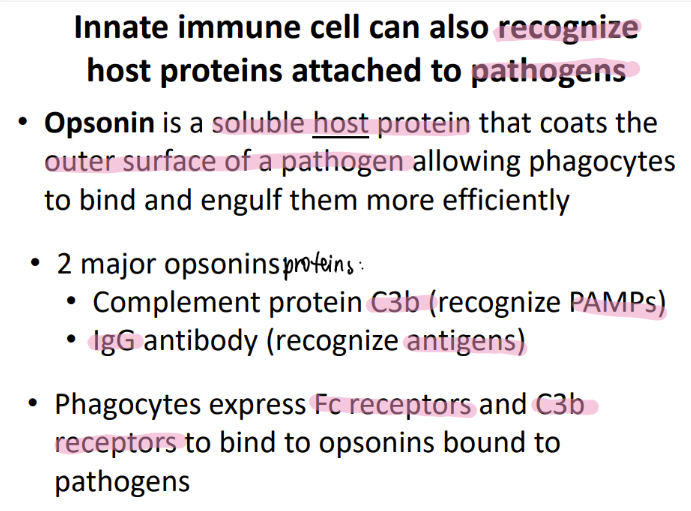
what are the 2 examples of opsonins?
complement protein C3b (binds to PAMPs, aids in recog)
IgG = actual antibody = (aids in recog antigens)
wtf do phagocytes do when they see C3b or IgG?
bind to them using Fc and C3b receptors!
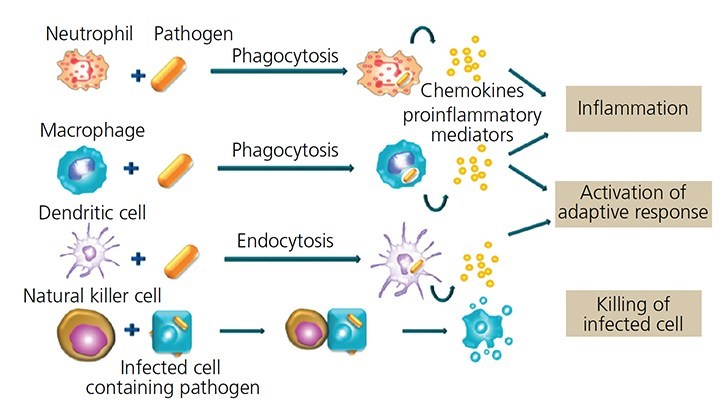
what are the 5 steps in accute inflamm?
recognize foreign/dead cells
release cytokines (inflamm mediator pro’s)
recruit WBC’s
remove bad guy via phagocytosis
clear away anything leftover+ heal
take a deep breath.
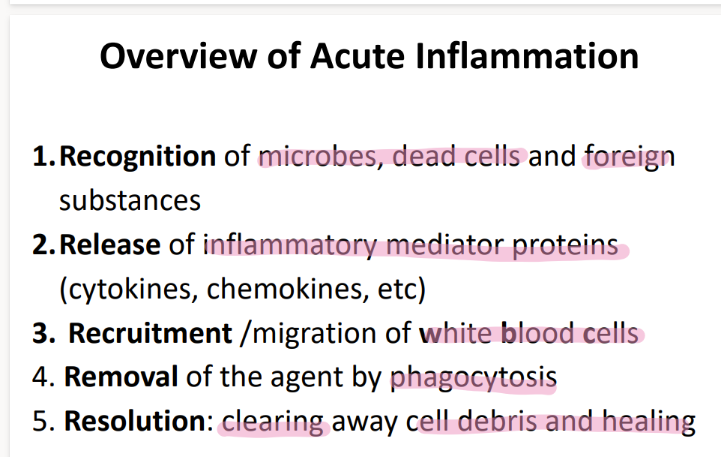
what are the first 3 steps that happen in acute inflammation?
MACROPHAGE releases vasoactive substances + cytokines
mast cell releases HISTAMINE (“degranulate”)
vasodilation babyyy
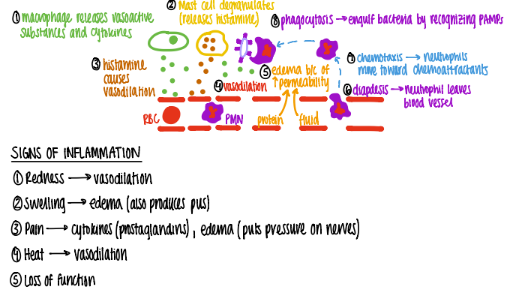
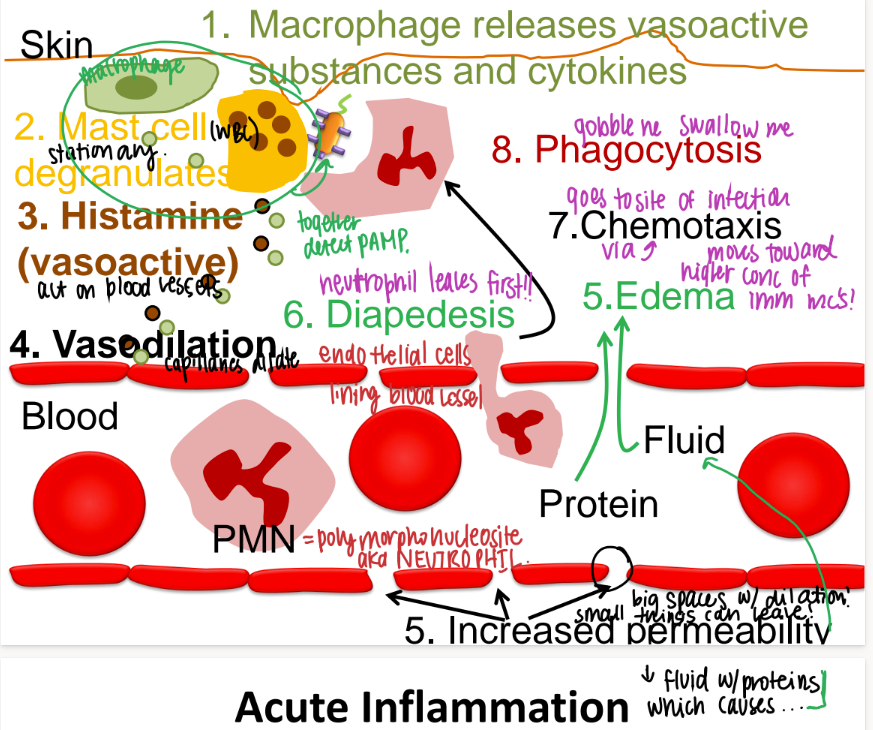
take a deep breath. what are the last 3 steps in acute inflammation, after vasodilation?
proteins, fluids, neutrophils (diapedesis) move towards chemoattractants bc of CHEMOTAXIS
swelling/ edema/ pus
phagocytosis bc recognize PAMPS.
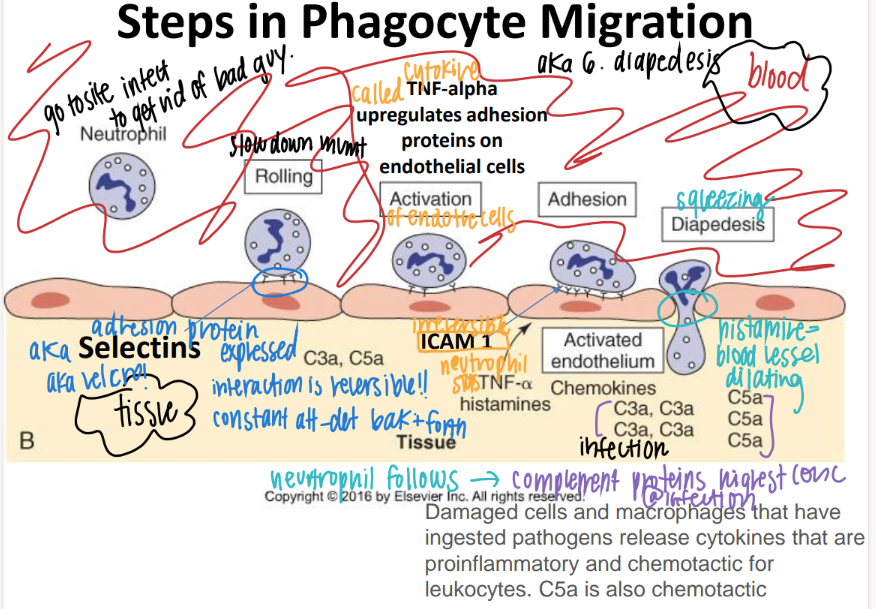
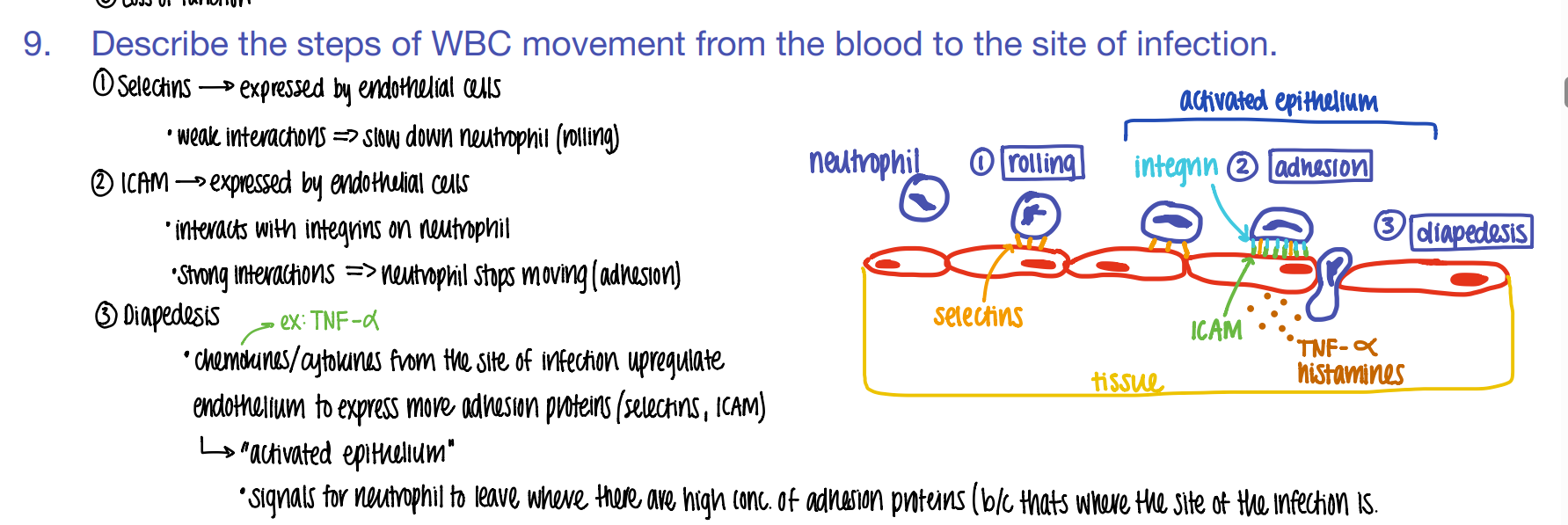
step 1: how tf do WBC’s get to the site of infection?
what is this process called?
ROLLING: selectins on endothelial cells slow down the neutrophil to ROLLING.
“phagocyte migration”
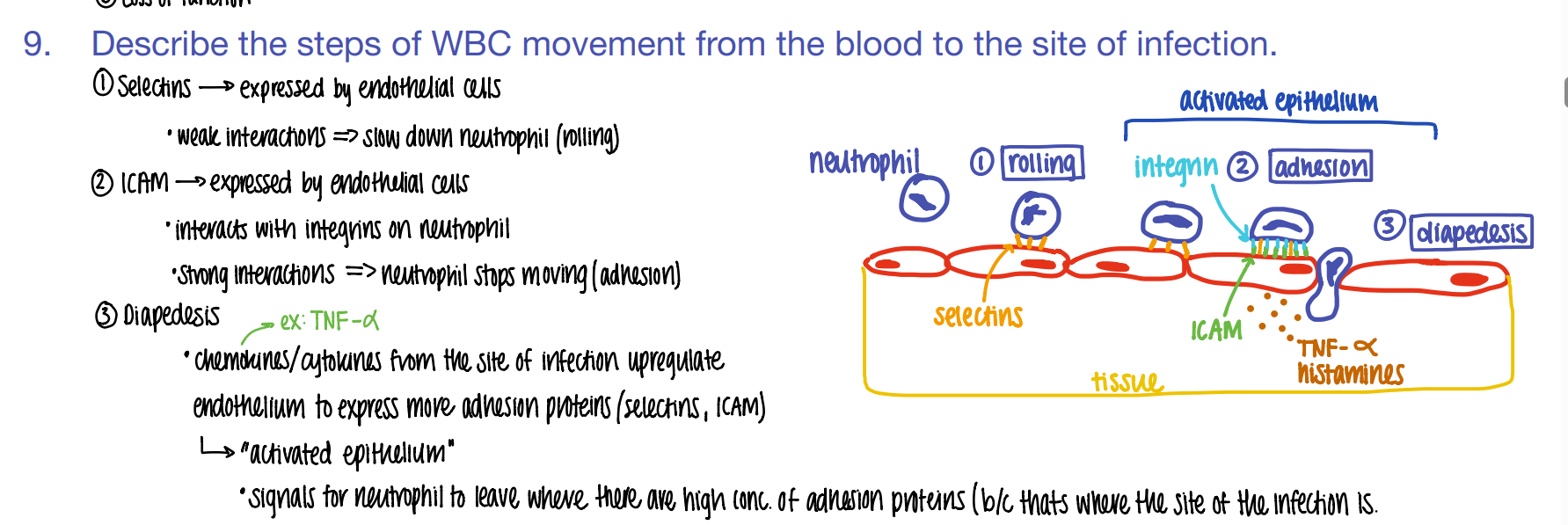
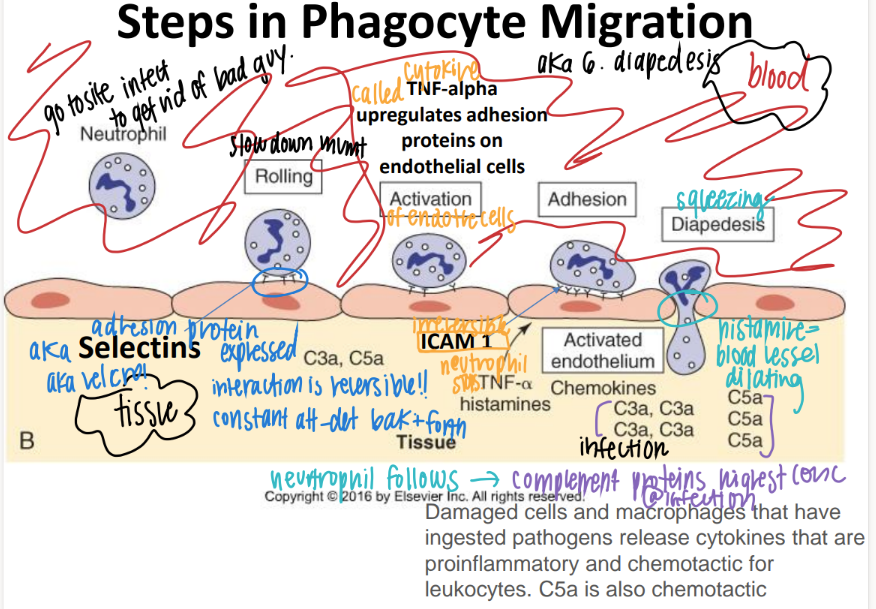
what’s the 2nd step of phagocyte migration?
ADHESION.
ICAM pro’s on endothe cell STICK to INTEGRIN pro’s on neutrophil
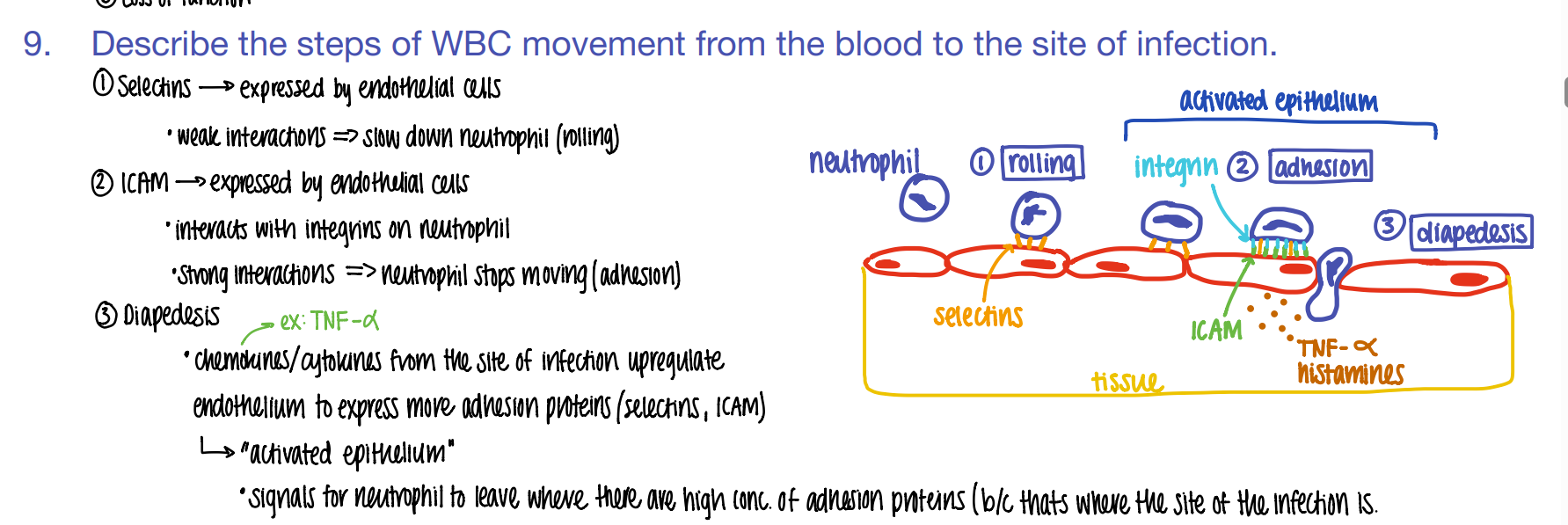
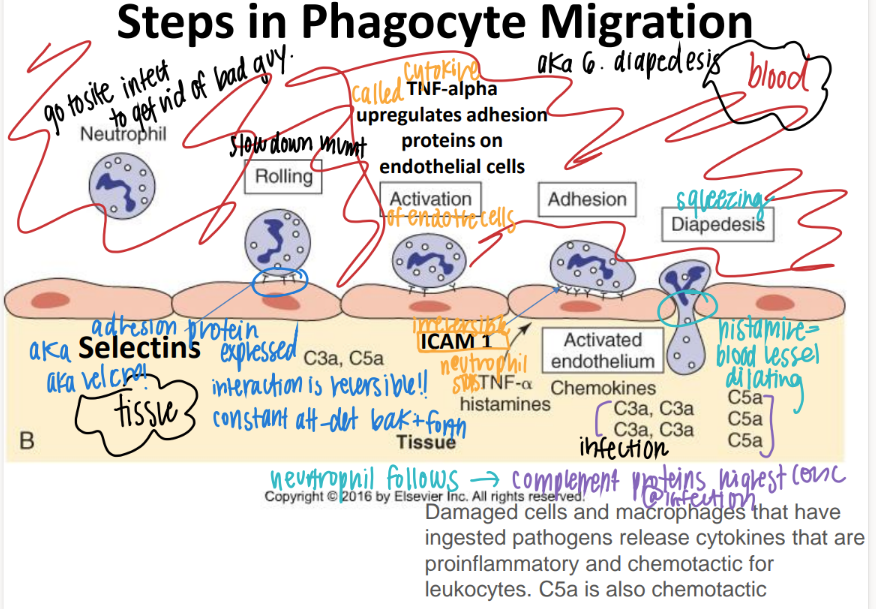
what’s the 3rd step of phagocyte migration?
DIAPEDESIS (WBC squeeze fr capillary to tissue)
BECAUSE TNF-alpha histamines / cytokines released FROM the site of infection cause MORE adhesion pro’s to be expressed which attracts MORE wbc’s!
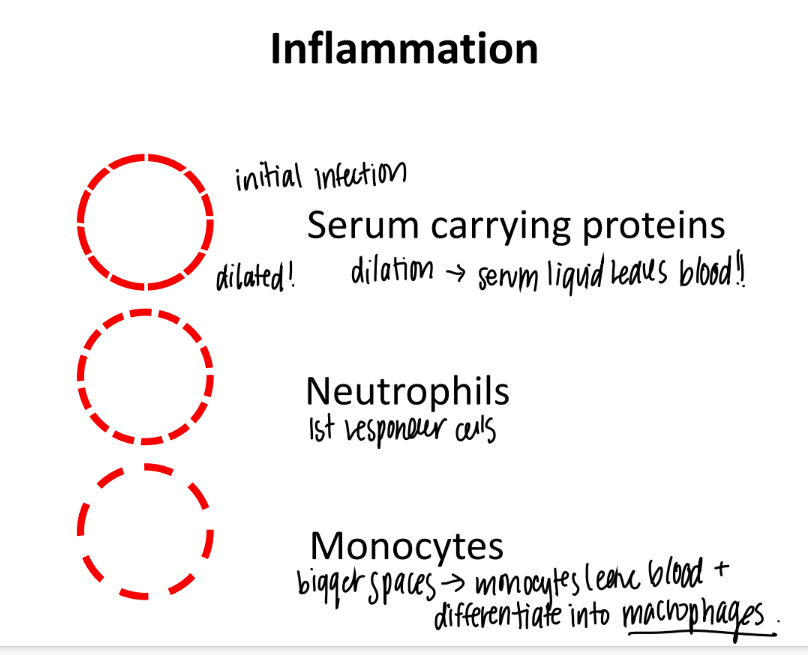
during inflammation, vasodilation gets bigger and bigger and bigger. what cells come first, then 2nd, then 3rd?
JUST serum w/ proteins
neutrophils
monocytes (which turn into dendritic cells + macrophages.
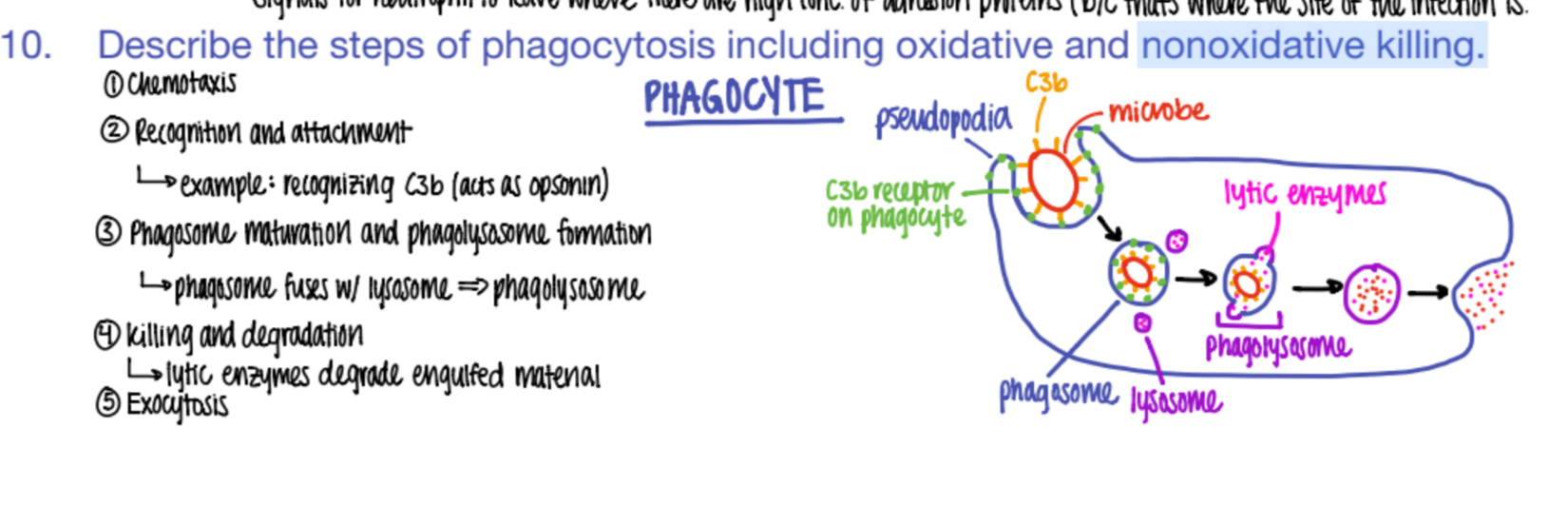
what are the 5 steps of phagocytosis?
chemotaxis (go to site of inflammation)
phagocyte recog + adhere to OPSONIN pro’s on pathogen.
phagosome + lysosome bind together = phagolysosome
digest babyyy (killing + degradation)
exocytosis
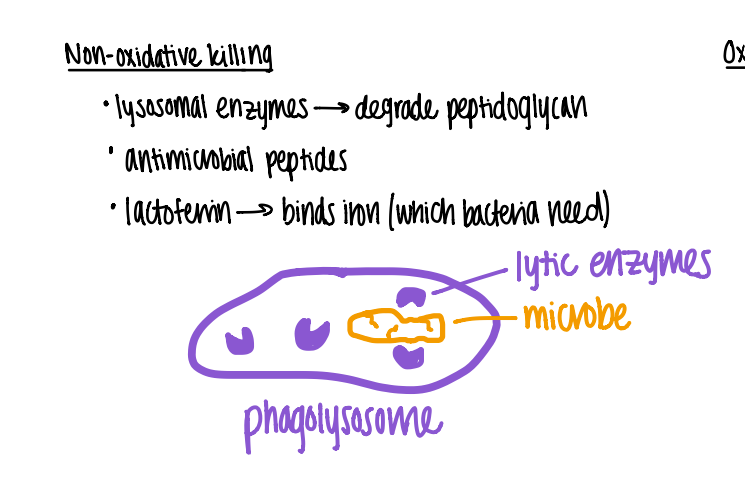
wtf is “nonoxidative killing” ?
what topic is it?
in the phagolysosome, the lyzosomal enzymes degrade peptidoglycan.
enzymes = lysozyme, phospholipase, proteases
phagocytosis

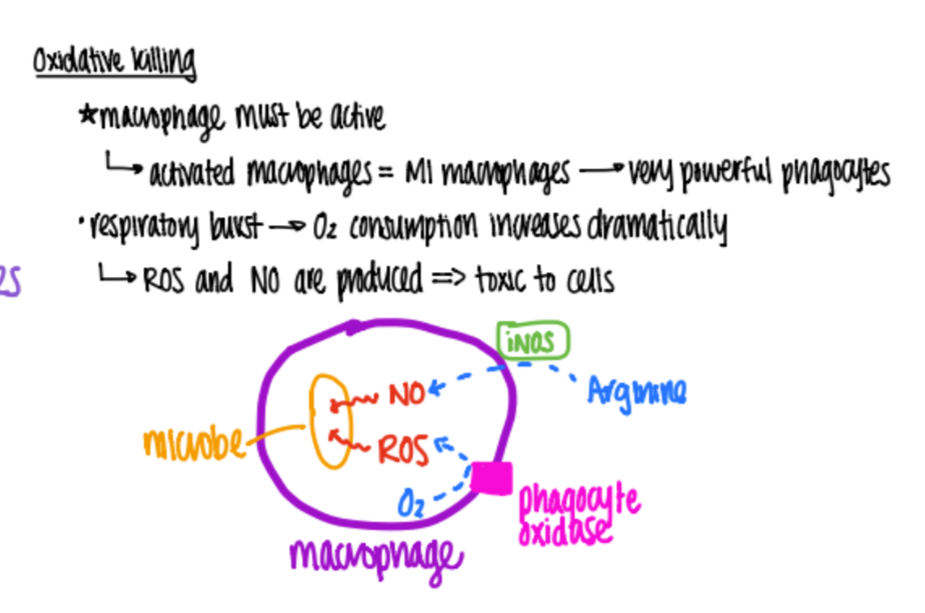
wtf is “oxidative killing” ?
phagocytes undergo “respiratory burst” = metabolize O2 into H2O2 (reactive O2 species ROS) which DEGRADE the bacteria.

what is the pH of the phagosome?
4-5

what cells do phagocytosis?
a. neutrophils
b. macrophages
c. dendritic cells
d. all of the above
d. all of the above!
what are some examples of cytokines?
chemokines, interferons, TNF-alpha, interleukins

what’s the purpose of cytokines?
who makes them?
do cytokines act systemically or locally?
released by WBC’s to regulate activity of OTHER wbc’s!
work BOTH locally AND systemically!
(blood and nearby!)

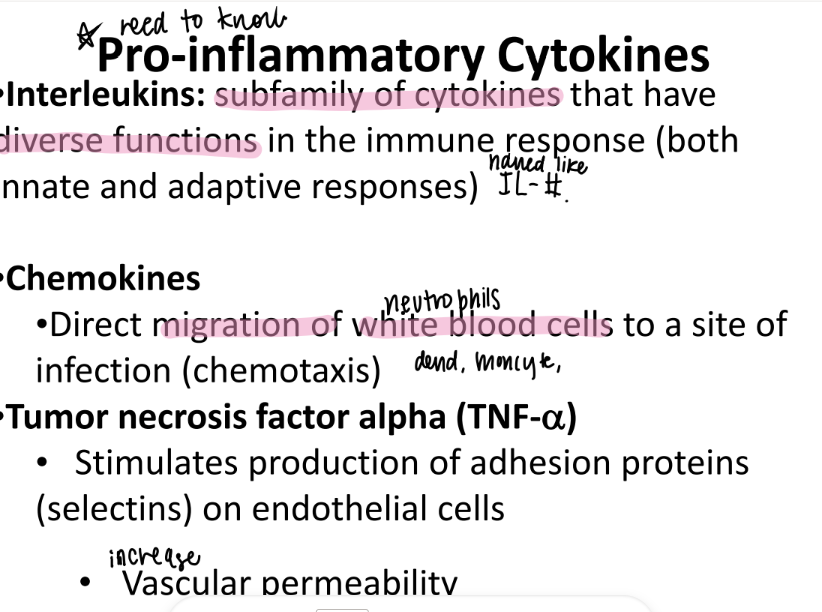
tf are “interleukins”?
pro-inflammation cytokines!
w/ diverse functions…
work in ADAPTIVE and immune res.
wtf are chemokines?
cytokines that ATTRACT WBC’s to infection site.
what does TNF alpha stand for?
what is it categorized as?
tumor-necrosis-factor-alpha cytokine
what does TNF alpha do?
STIMULATE production of SELECTIN adhesion pro’s on endothe cells
(which does “rolling” of wbc’s).
so…
2. INCREASE vascular permeability
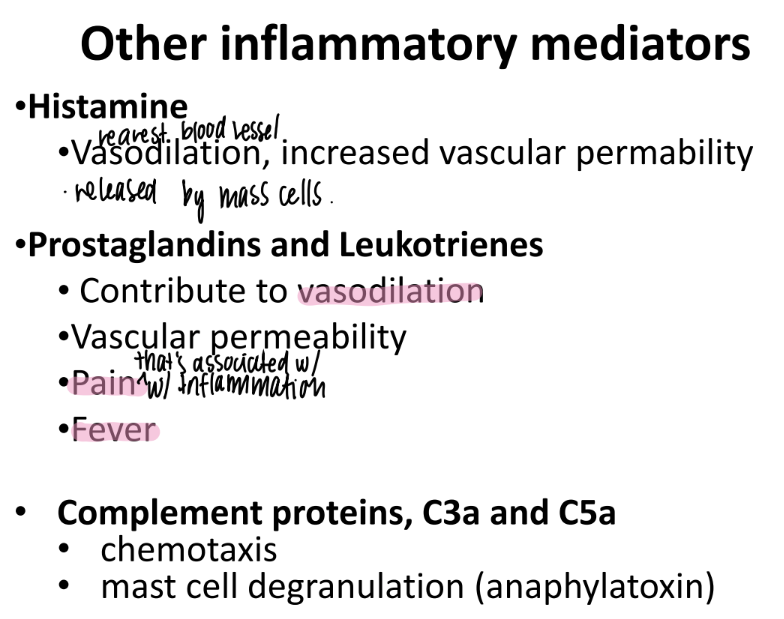
what does histamine do?
what is it made by?
incr VASODILATION + “vascular permeability”
made by MAST cells (innate imm)
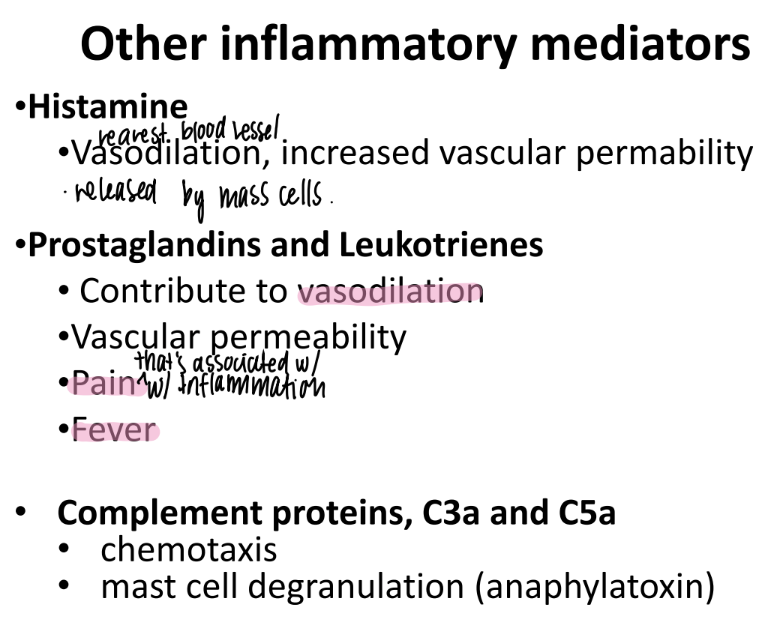
wtf do prostaglandins + leukotrienes do?
incr VASODILATION + vascular permeability
responsible for PAIN + FEVER
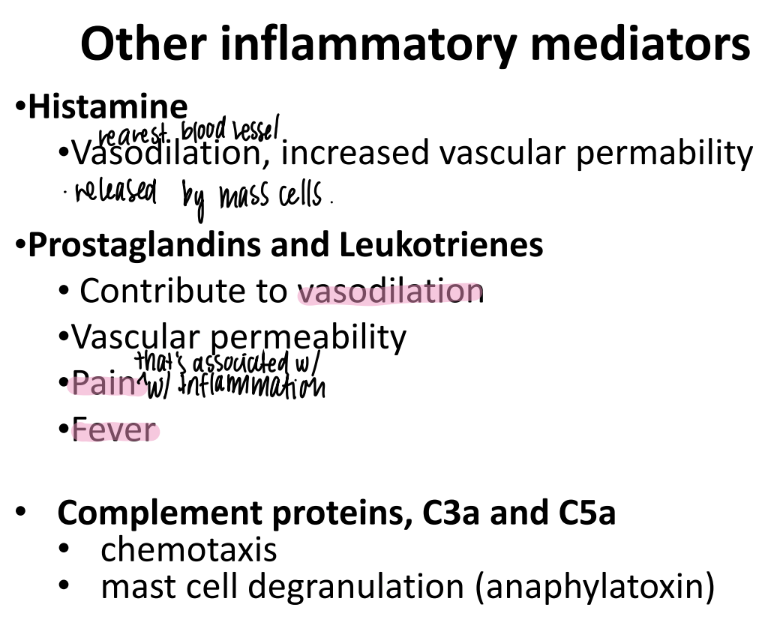
What do C3a and C5a do?
complement proteins that activate chemotaxis (mvmt of WBC’s to injury)
promote MAST CELL de-granulation
mast cells have pro-inflamm stuff in GRANULES ie histamine.
anaphylatoxins → sim to allergic rxn
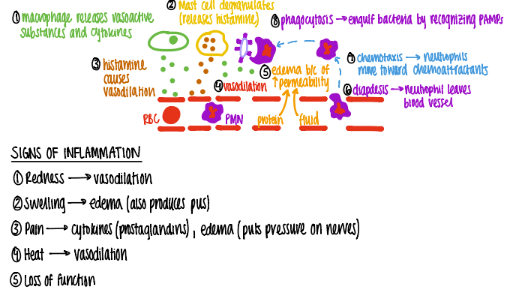
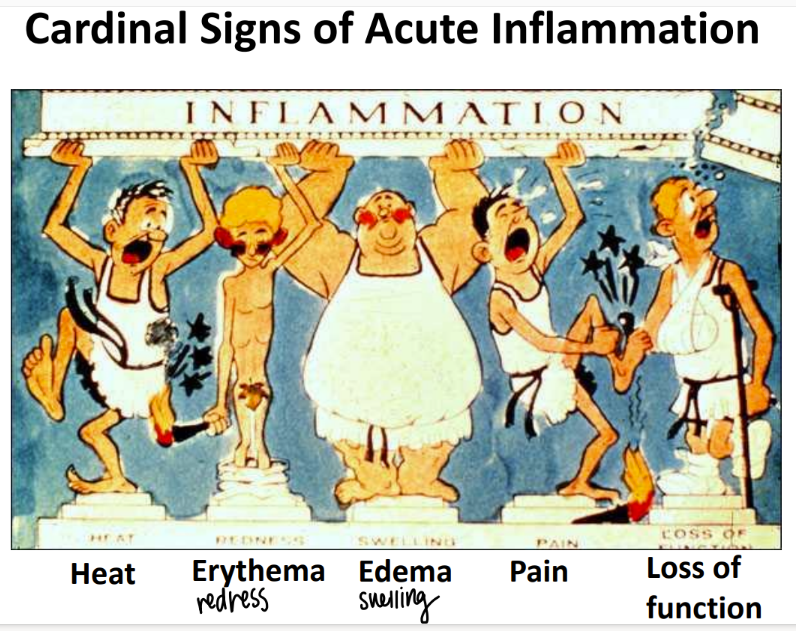
what are the 5 signs of acute inflammation?
heat
redness (erythema)
swelling (edema)
pain
loss of function
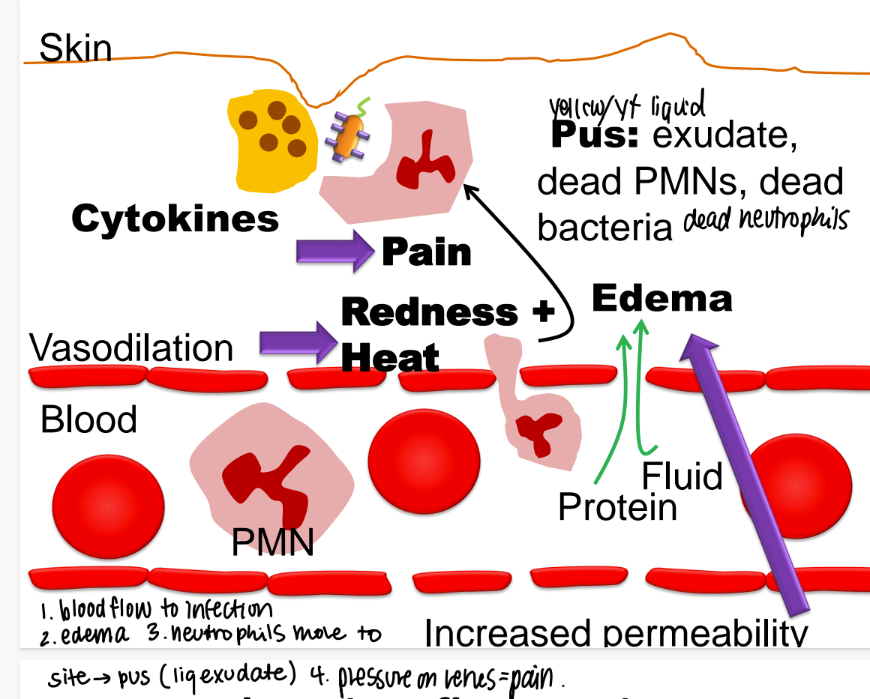
what happens in chronic inflammation?
incr blood flow to infection
swelling (edema)
neutrophils move to the site → PUS (exudate)
pressure → pain, redness
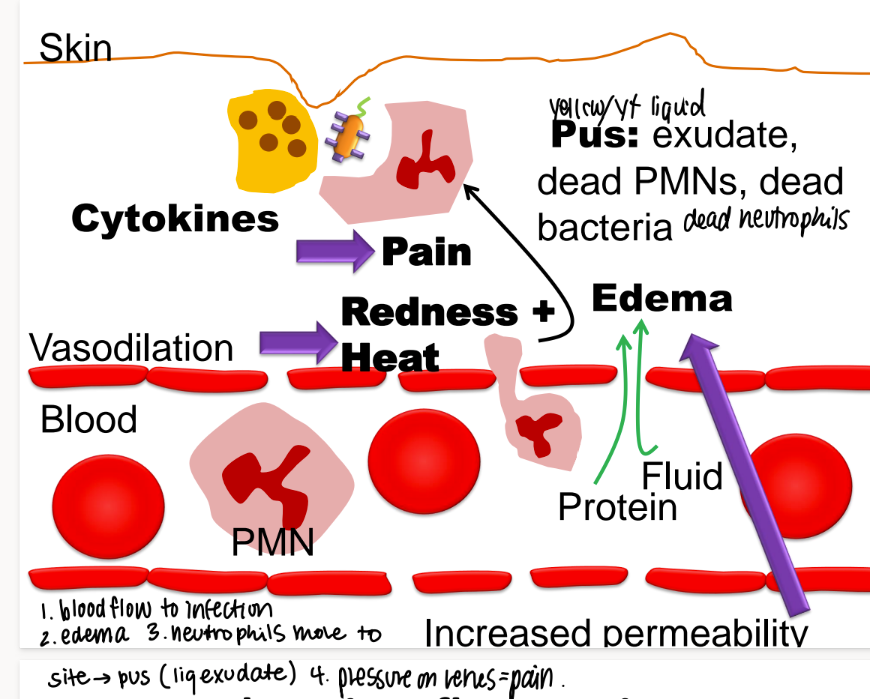
what mc’s are in pus?
exudate
dead neutrophiles/ PNMs
dead bacteria
(exudate is any fluid that OOZES out of tissues)
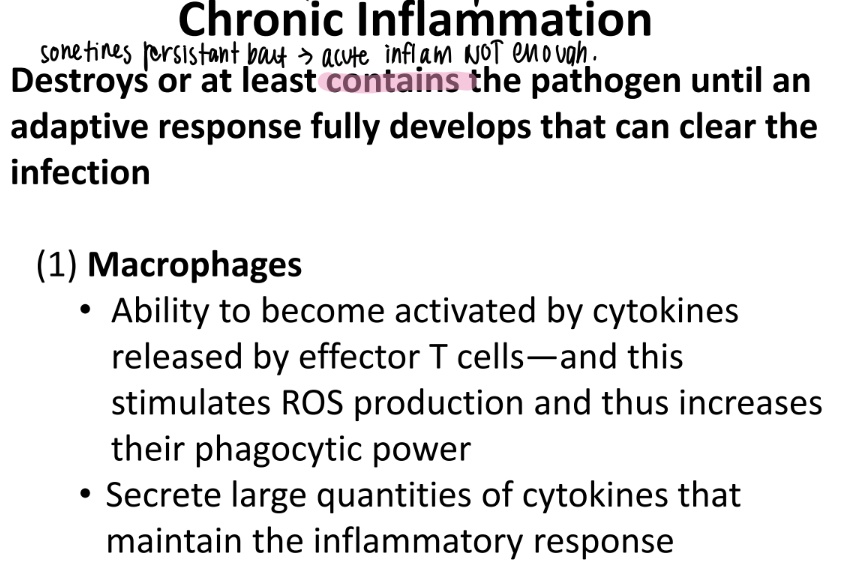
how are macrophages activated?
then what do they release?
effector T cells release cytokines to…
activate macrophages → release ROS + MORE cytokines that MAINTAIN the inflamm
what do lymphocytes do in chronic inflammation?
adaptive or innate imm?
T and B cells DIRECT attack against invincible pathogens
ADAPTIVE imm

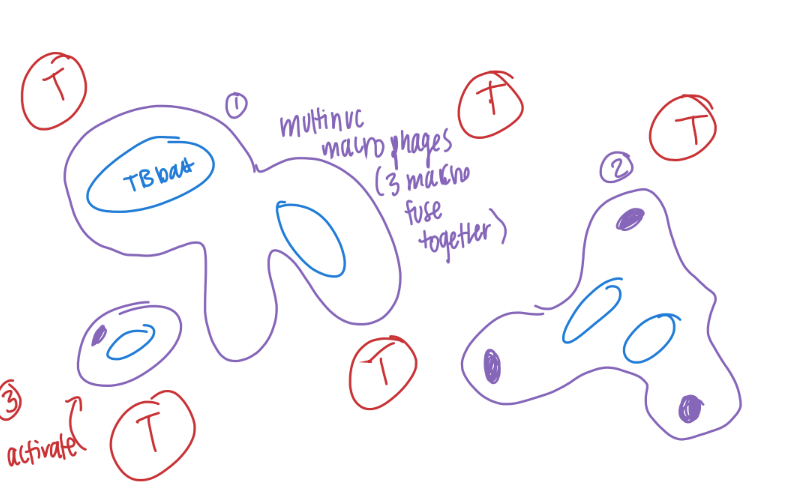
how are granulomas formed/ what are they made of?
what do they do?
macrophages FUSE together to make GIANT MULTINUCLEATED cells.
combine w/ macrophages + T cells
to WALL OFF (contain) invincible pathogens (ie mycobacterium TB)

what is the con of granulomas?
accumulate = INTERFERE with NORMAL tissue funct.
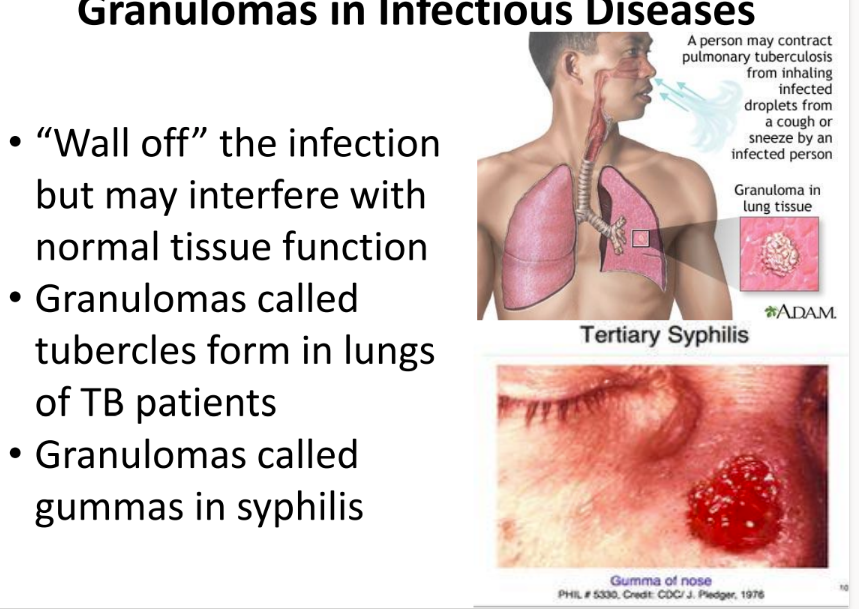
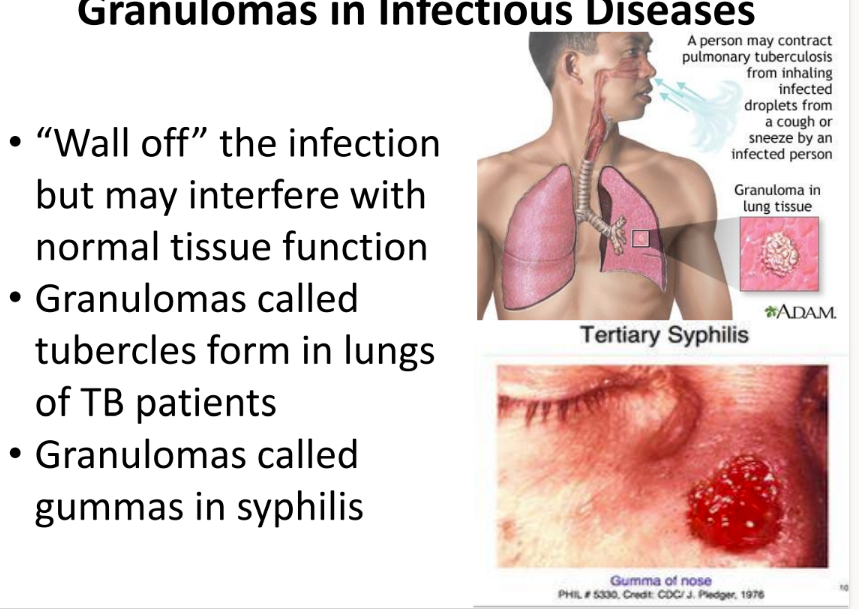
what are 2 examples of problematic granuloma clusters in infectious diseases?
tubercles = lungs of TB pts
gummas = syphilis
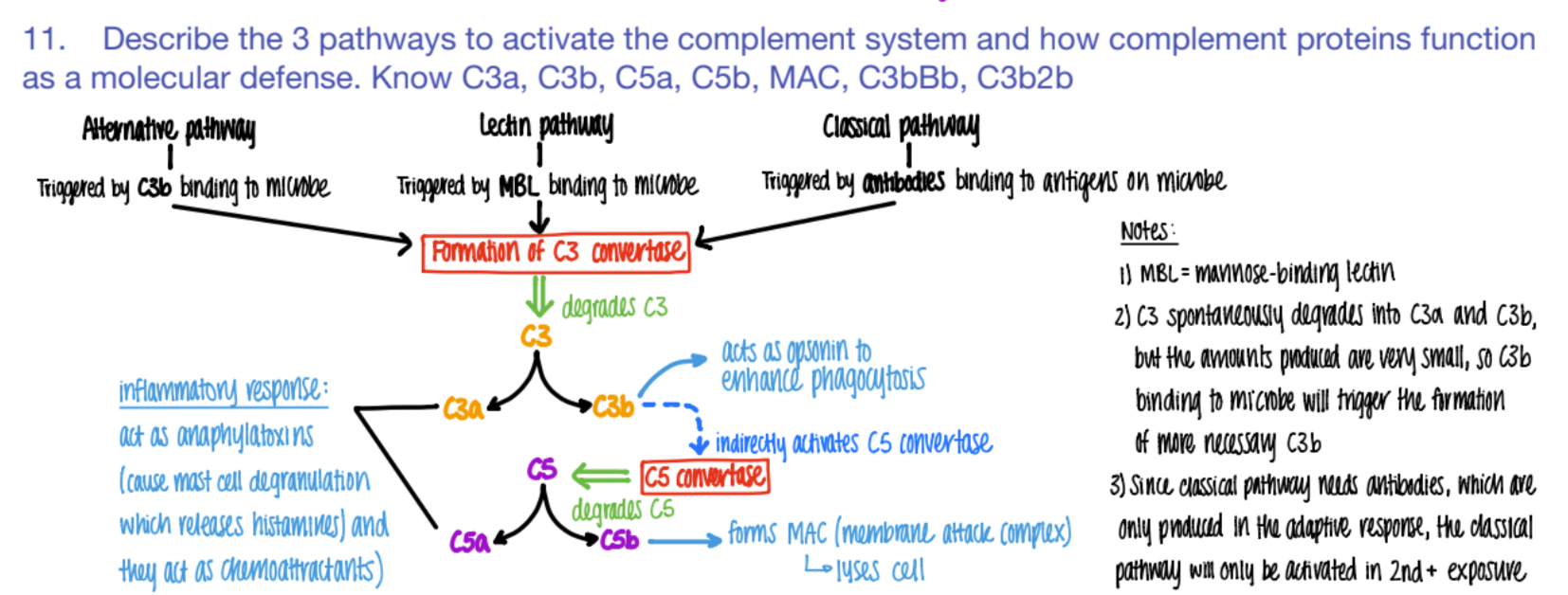
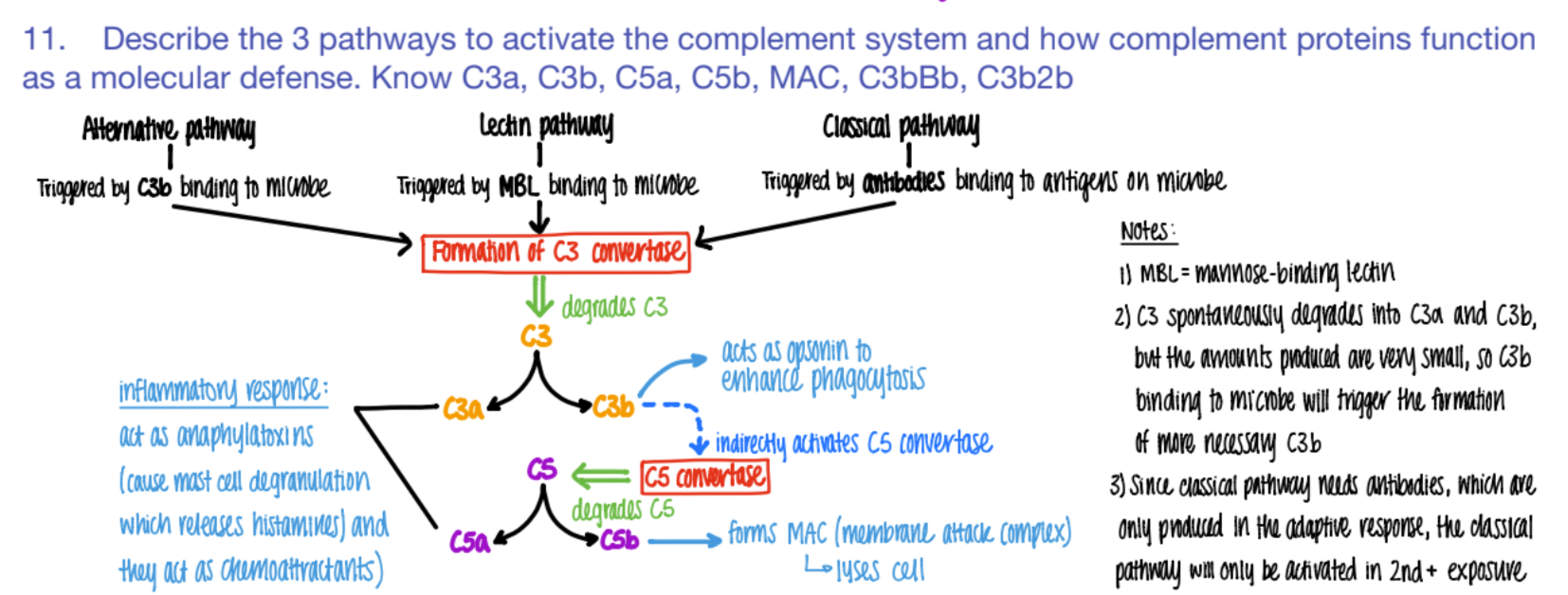
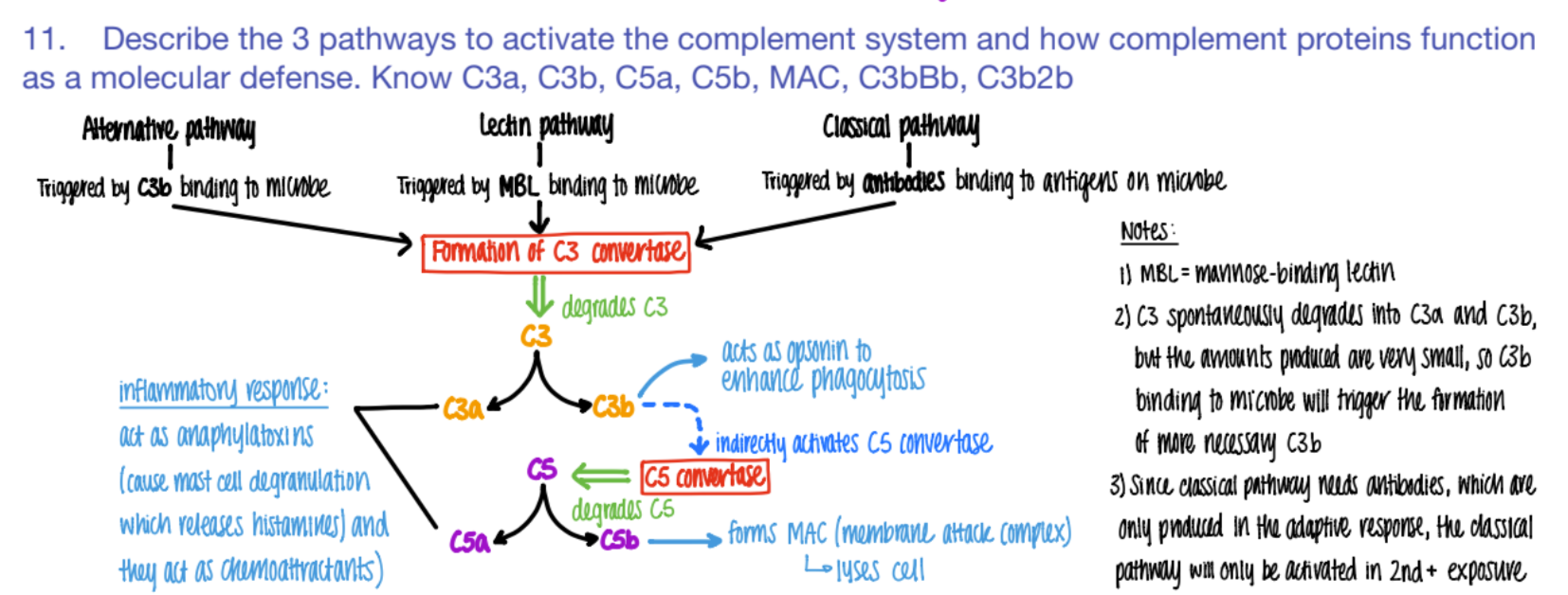
what activates the CLASSICAL pathway of the complement system?
what does it cause?
antibodies bind to ANTIGENS (on microbe)
C3 convertase makes C3a and C3b.
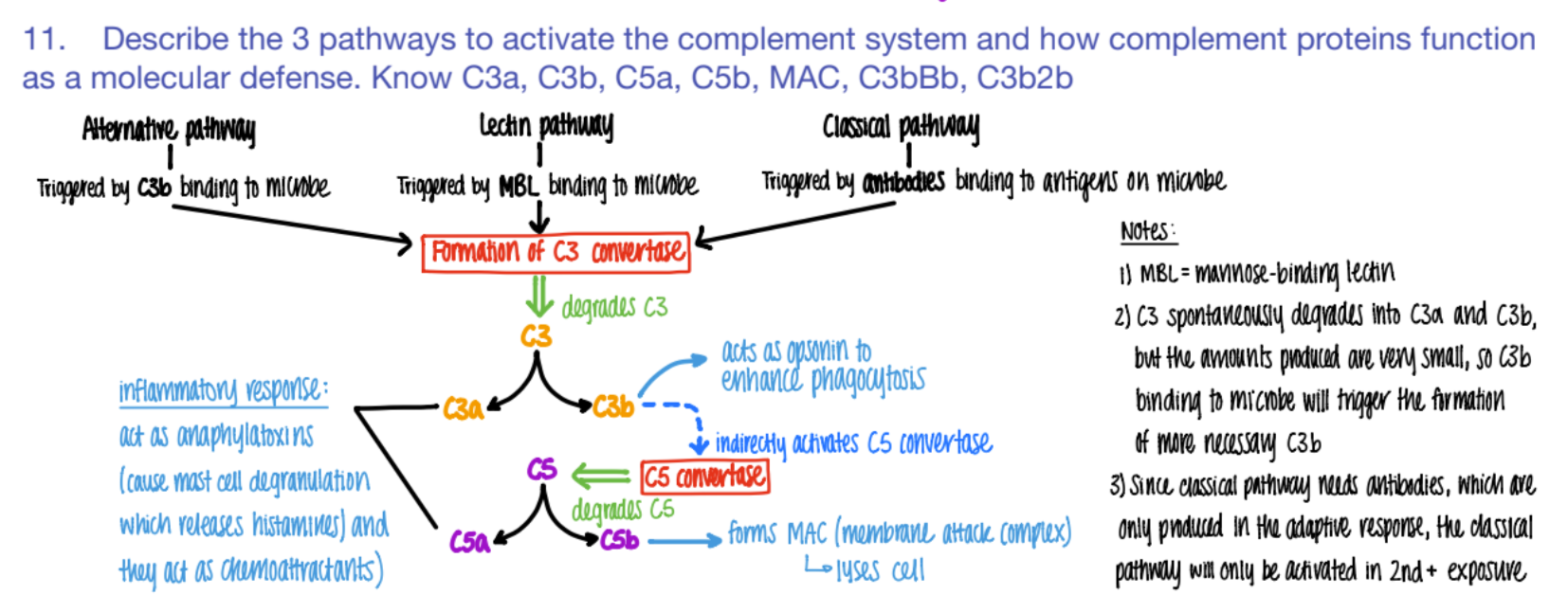
The classical pathway is only activated if…
why?
SECOND exposure
bc ANTIBODIES formed in the ADAPTIVE response!
it’s CLASSIC for antibodies to bind to antigens.
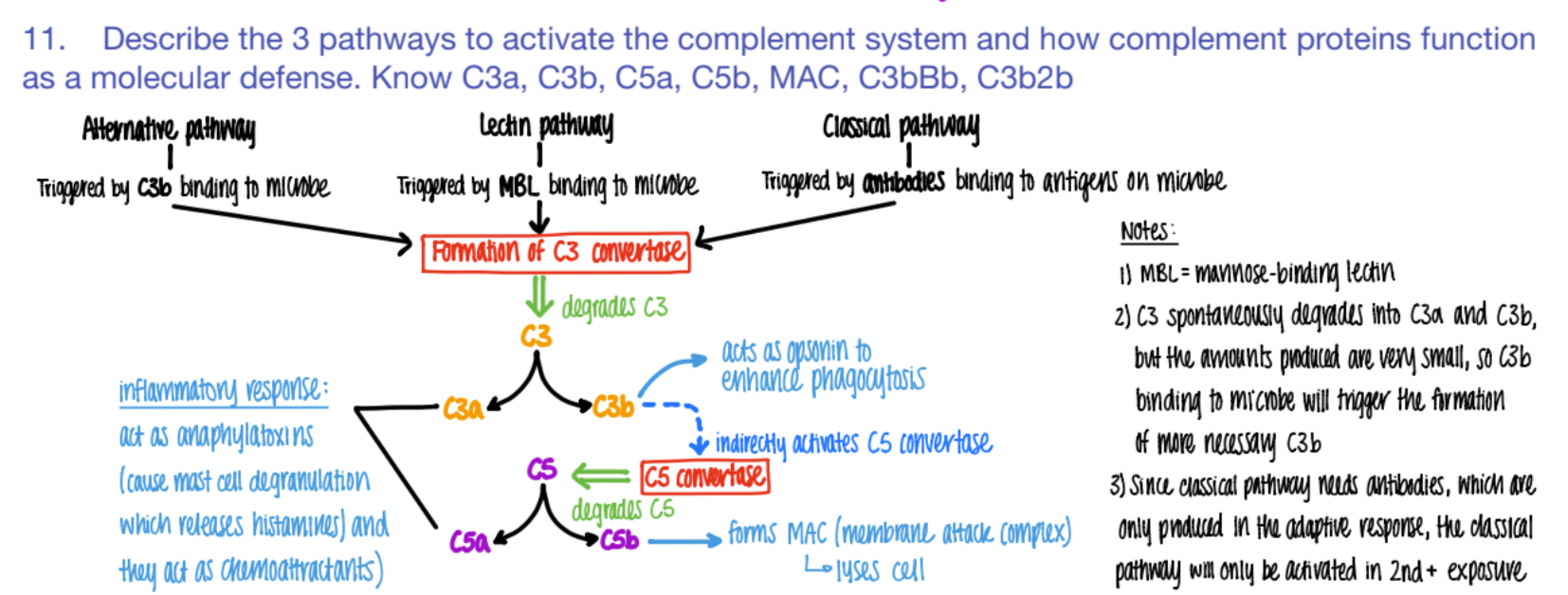
what activates the LECTIN pathway of the complement system?
what does it cause?
MBL (mannose binding pro) binds to PROTEIN
C3 converase makes C3a + C3b
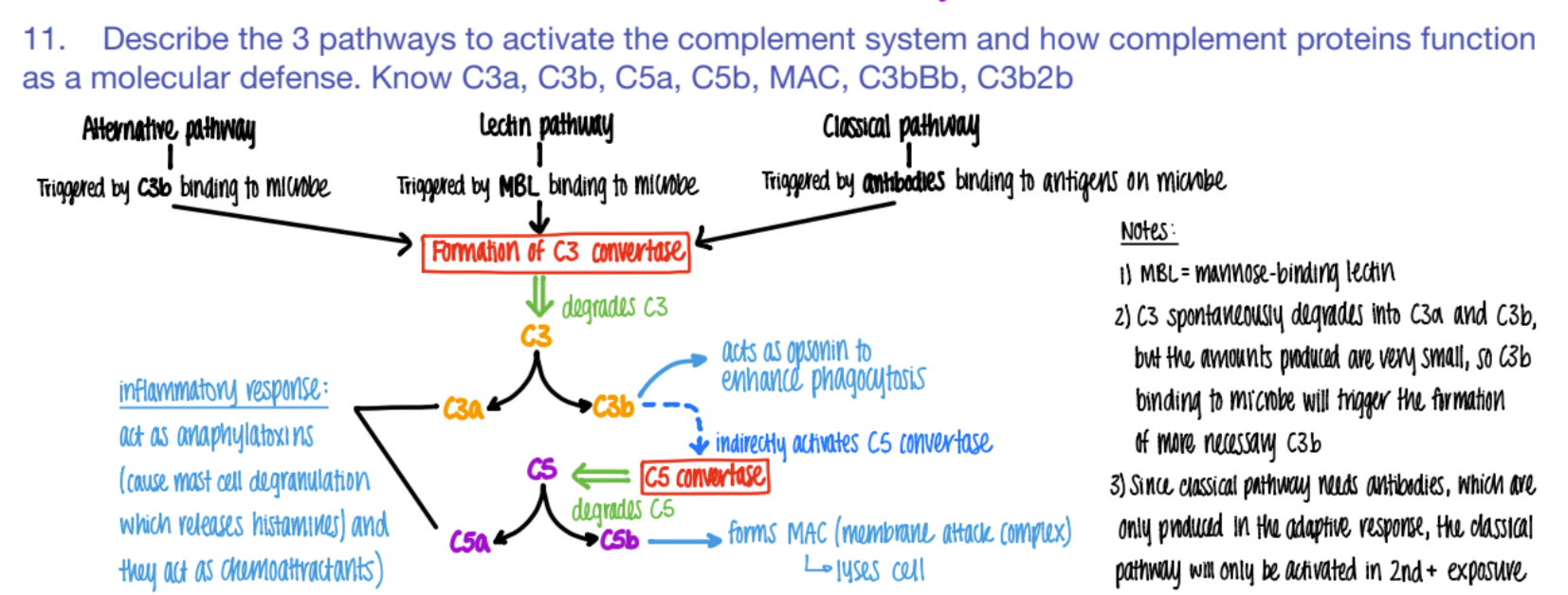
what activates the ALTERNATIVE pathway of the complement system?
What does it cause?
C3b binds to microbe
c3 convertase makes C3a + C3b
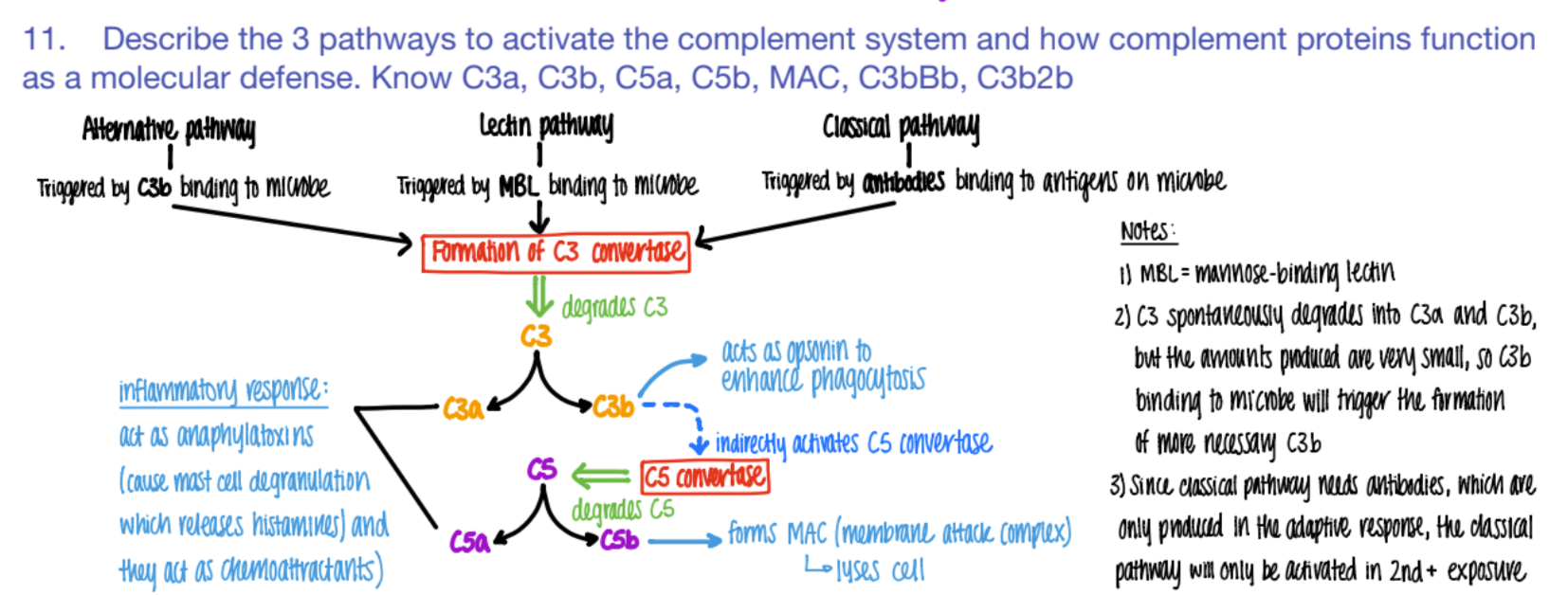
wtf do C3a and C5a do in the complement system?
INFLAMMATION!
anaphylaxotoxins (histamine from mast cells de-granulating)
chemoattractants
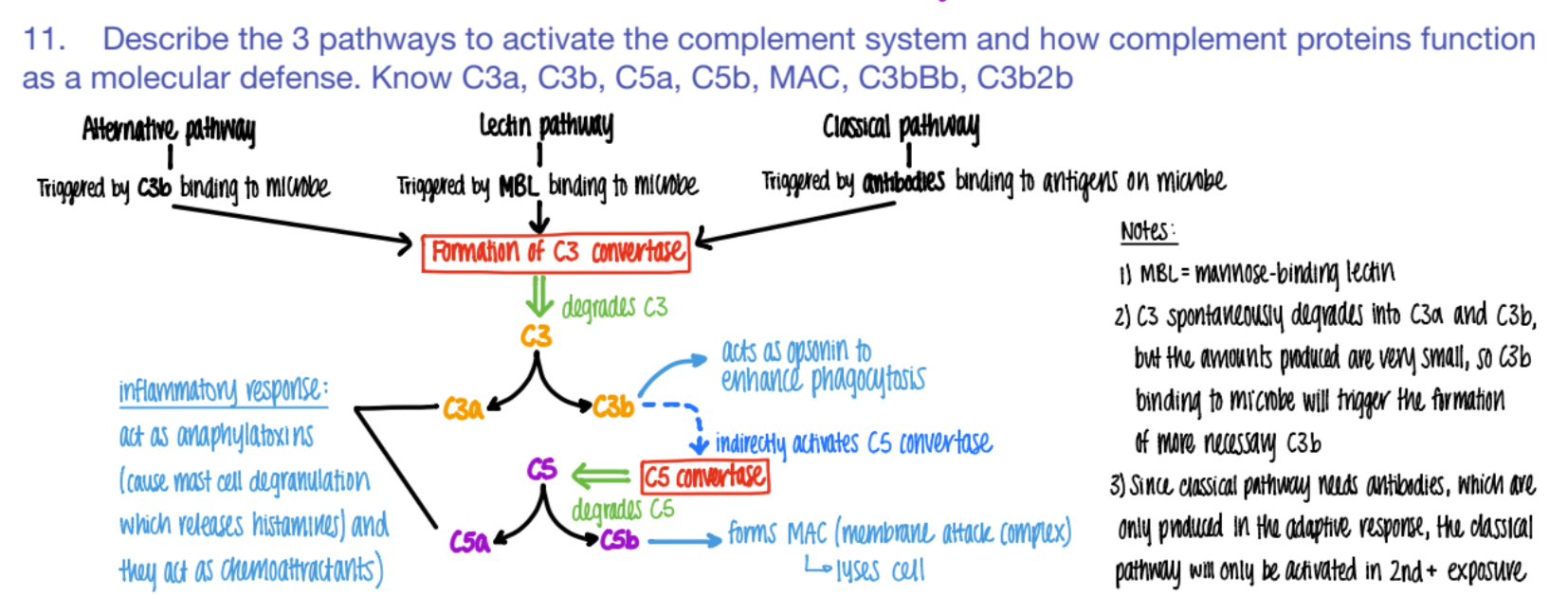
wtf does c3b do?
opsonin (so more c3b is made = better phagocytosis)
activates C5 convertase (→ more C5b
how does the complement system “bridge” the innate and adaptive imm system?

wtf does C5b do?
forms MAC
(C5b complex (C6, C7, C8, C9)
(membrane attack complex) to LYSE pathogen.
C5b complex (C6, C7, C8, C9) does WHAT?
it’s an MAC (membrane attack complex) that creates PORES in pathogen cell mem’s → lysis
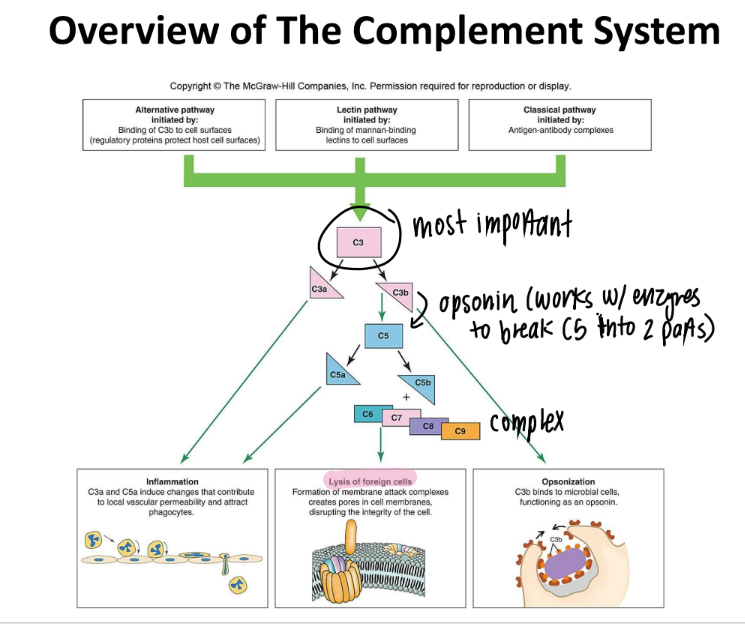
overall, what are the 3 results of the complement system?
inflammation (c3a c5a)
lysis (c5b MAC)
opsonization (c3b)
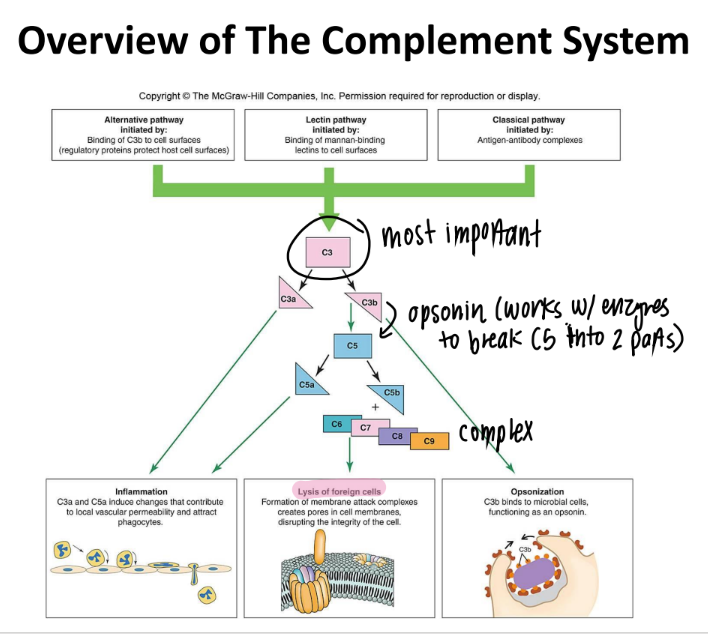
if you have a defective complement system, which protein is most likely affected?
c3!!!
cause it makes c3a, c3b, and activates c5s
what induces fever?
pyrogens!!
(exogenous OR endogenous)
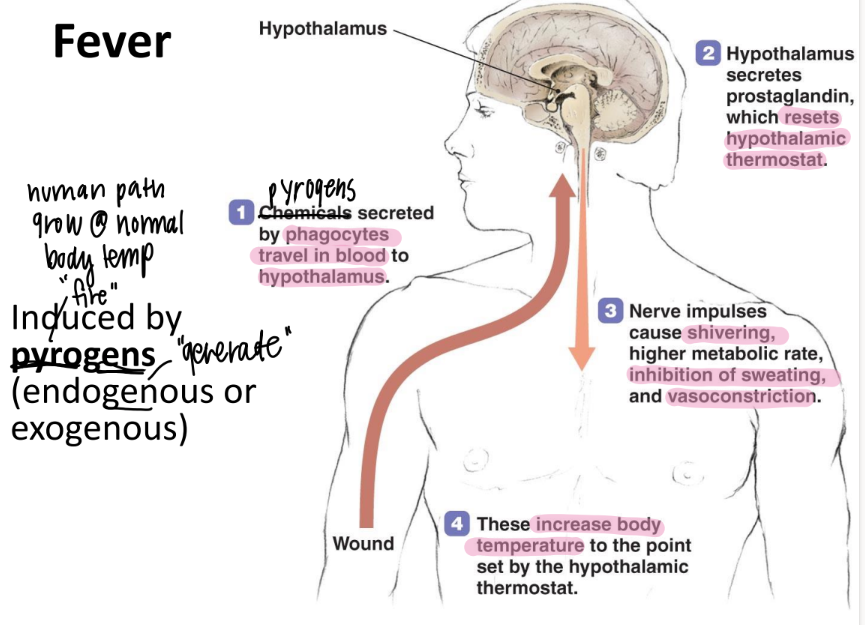
what are the 3 steps of fever induction?
pyrogens to hypothal
release prostaglandins → new set point
incr temp: shiver, vasoconstriction, higher metabolic rate, STOP sweating
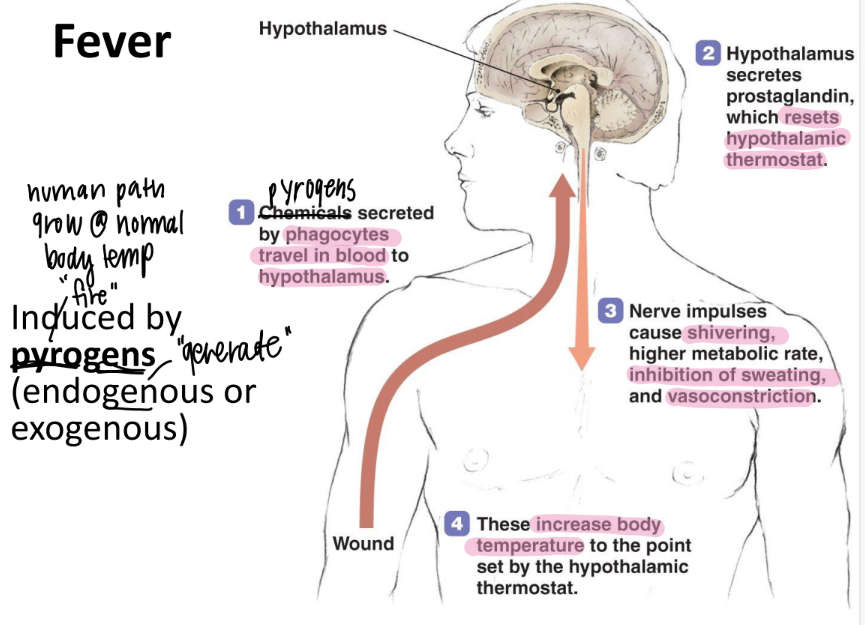
what are the benefits of fever?
inhibit high-temp microbes
WBC’s can move easier → faster phagocytosis + tissue repair

are comp pro’s + phagocytes good for viruses? why or why not?
HELL NO.
comp pro’s (ie phagocytes) canNOT get into the cell to kill the viruses specifically.
only kill OUTSIDE (extracellular) → unecessary inflamm

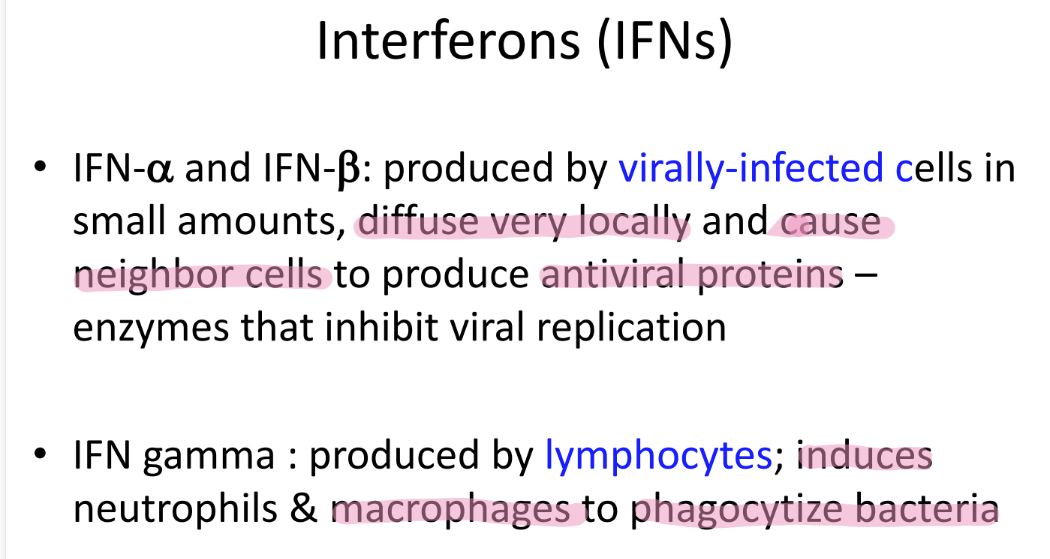
wtf are IFN alpha + beta?
who makes them?
what do they do?
proteins SECRETED by viruses (albeit only a bit)
that alert nearby cells to make ANTIVIRAL pro’s to inhibit viral rep
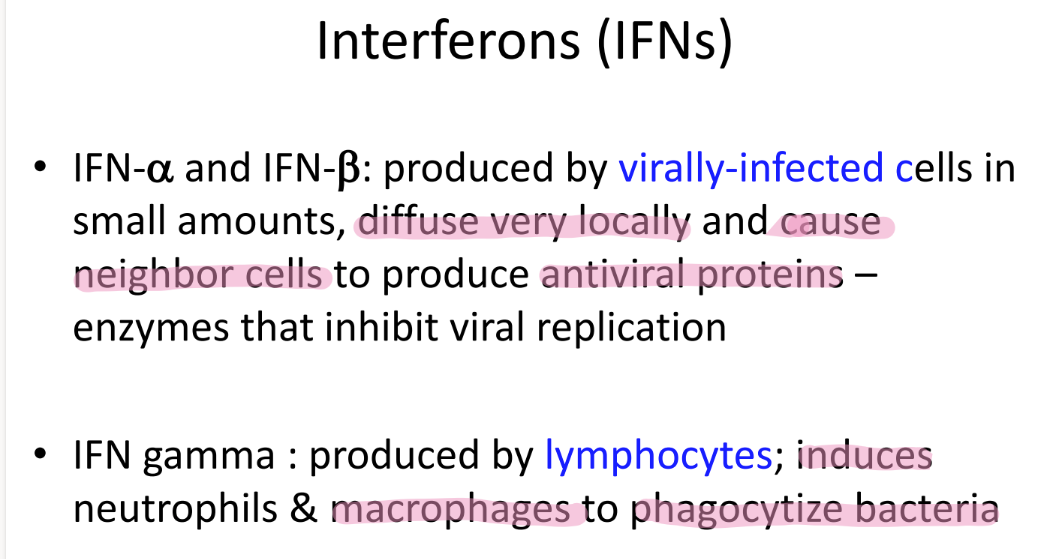
wtf is IFN gamma?
who makes them?
what do they do?
pro made by LYMPHOCYTES
induces neutrophils + macrophages to PHAGOCYTIZE bacteria
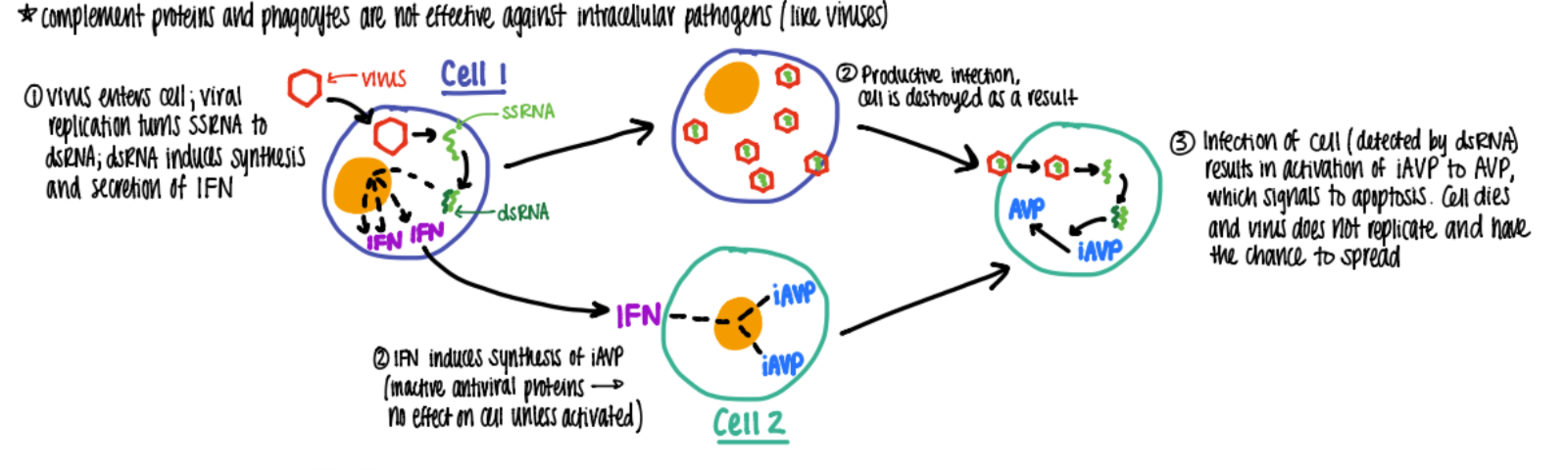
what’s the function of IFN A & B?
cause cell to make AVP
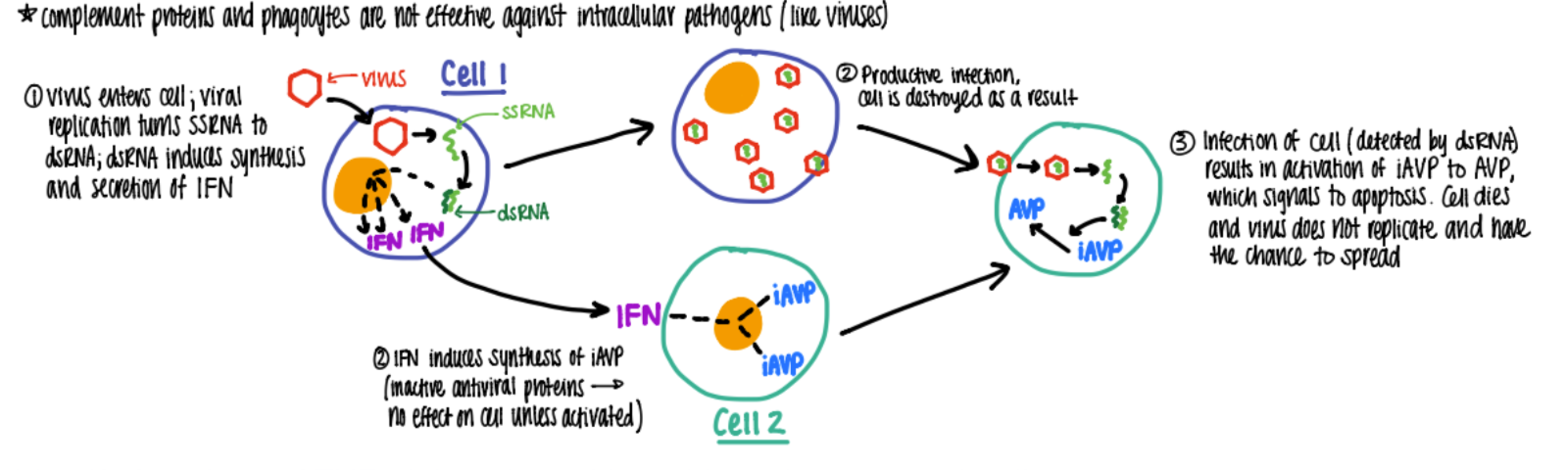
what’s the interferon alpha and beta pathway?
viral rep: ssRNA → dsRNA which makes IFN a+b
IFN tells nearby cells to make INACTIVE AV pro (antiviral)
(alt virus destroys host cell)
virus tries to attack cell w/ IFN→ AVP activated → cell does apop!
(summary: virus rep → a+ b → apop in infected cell)
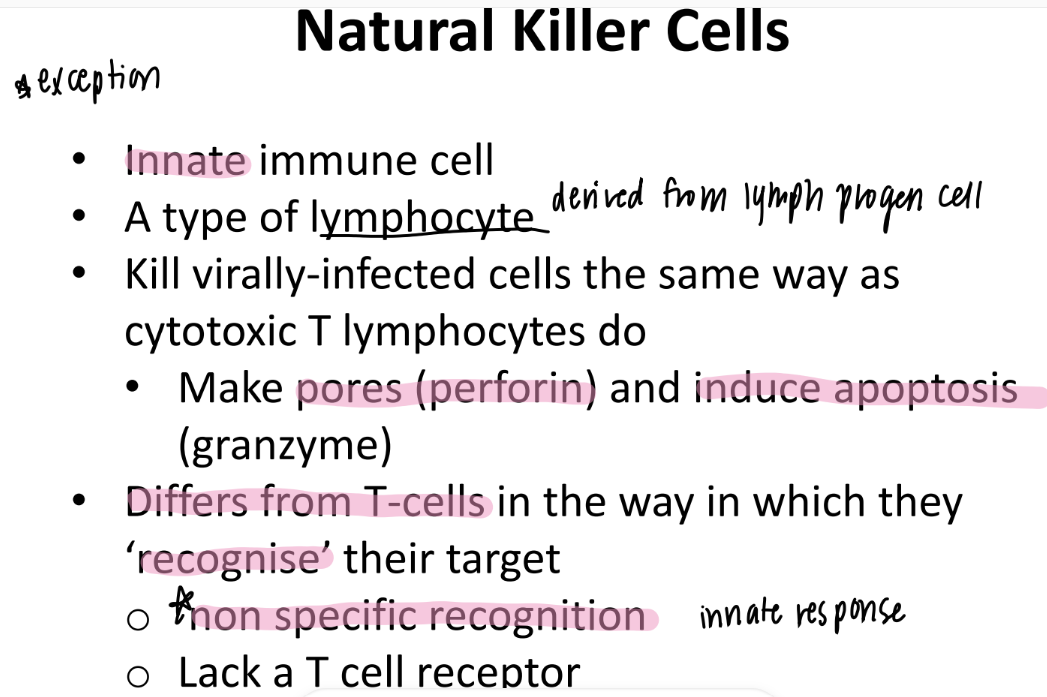
how are natural killer cells DIFFERENT from T cells?
NK = innate = NON-specific recognition = no T cell receptor
T cells = adaptive
how long does it take for adaptive immunity to kick in?
7-10 days
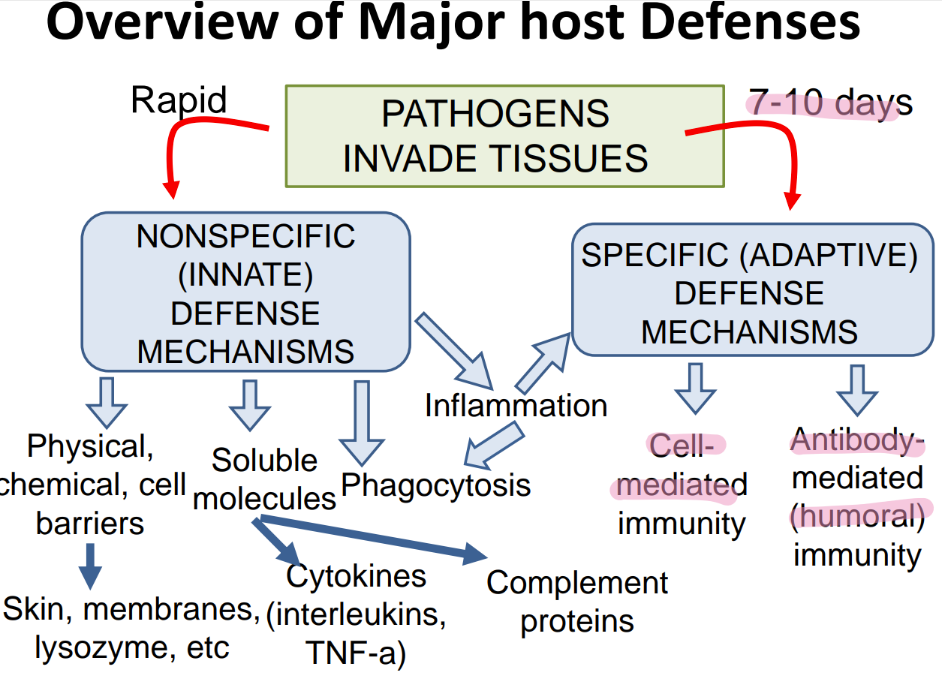
what are the 2 branches of adaptive immunity?
cell-mediated
ANTIBODY-mediated (humoral)
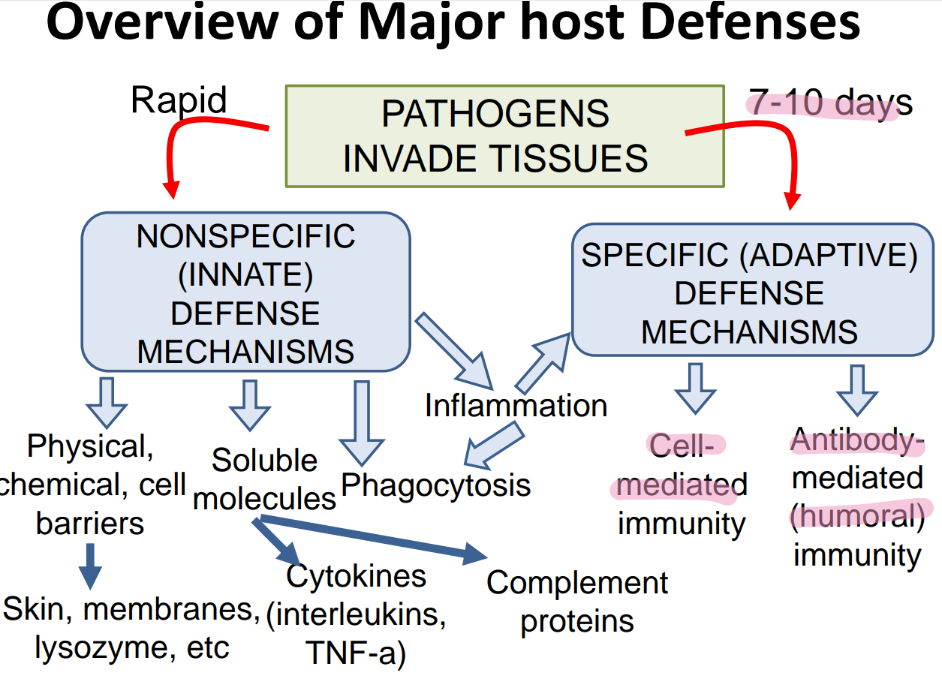
what does the inn recog in a pathogen, versus adaptive?
inn: PAMPs
adaptive: ANTIGENS
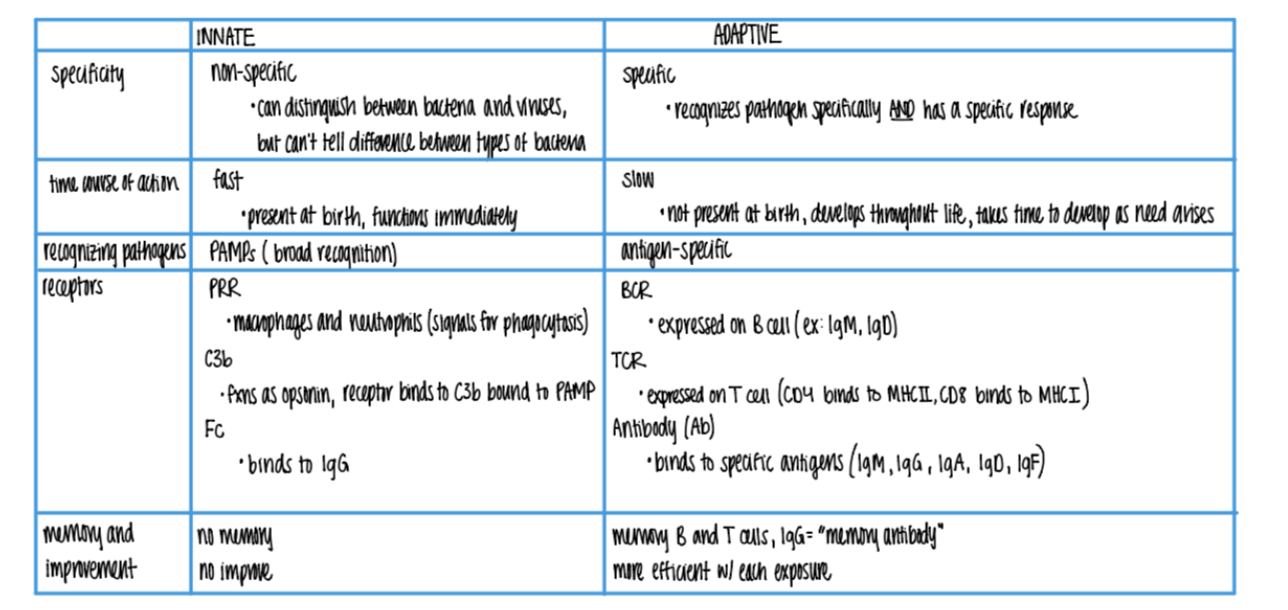
what is more SPECIFIC about the adaptive imm sys vs innate?
can distinguish btw BACT or viruses
can’t tell dif btw TYPES of bacteria
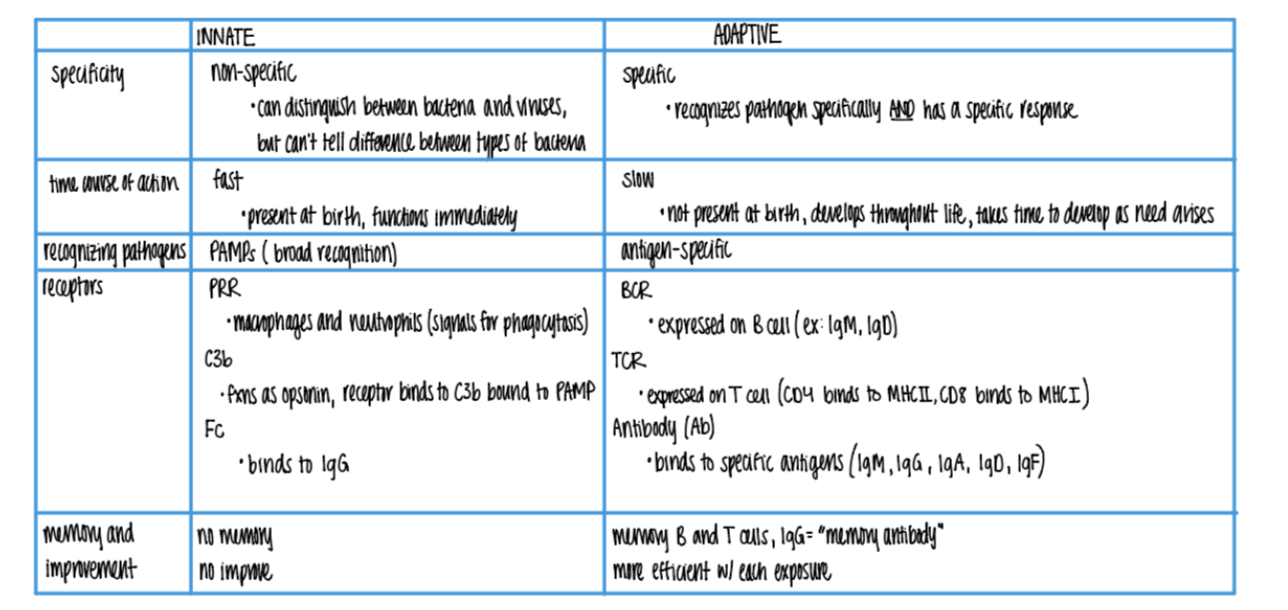
what’s the dif in TIMELINE btw innate + adaptive?
innate: FAST, made @ birth
adaptive: SLOW (wait 7-10 days), develops over LIFE
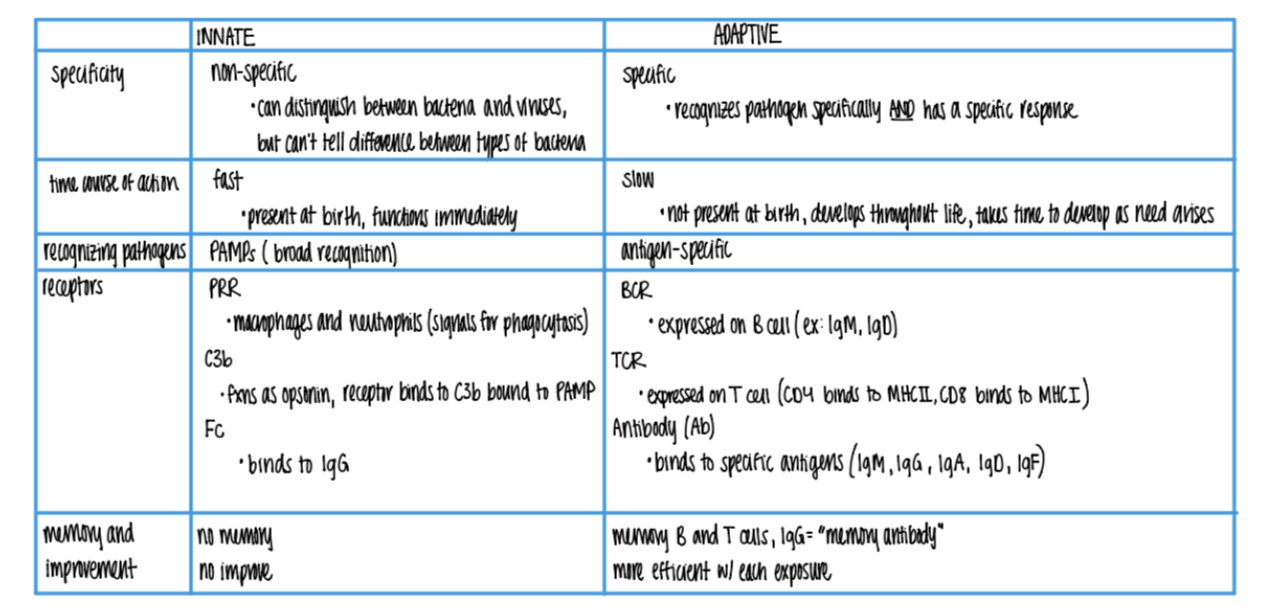
what are the 3 types of receptors found on wbc’s in the innate imm sys? what do they bind to?
PRR on macrophages + neutrophils → phagocytosis
C3b rec binds to C3b OPSONIN on PAMPs
Fc rec binds to IgG (antibody) opsonins on ANTIGENS.
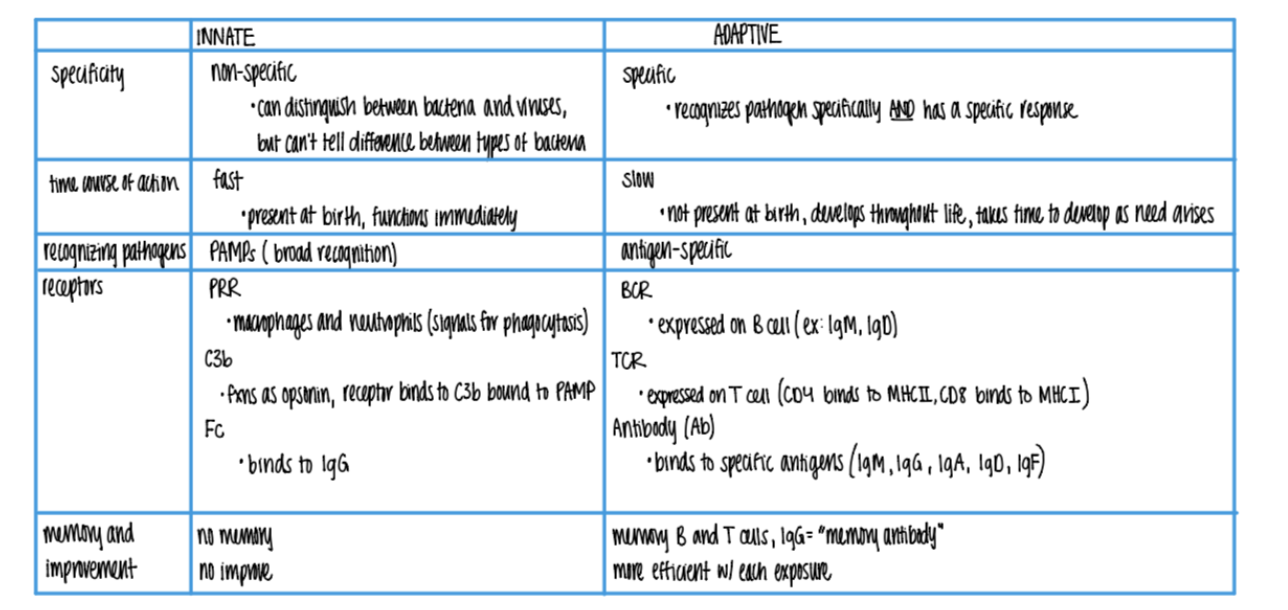
what are the 3 types of receptors found on wbc’s in the ADAPTIVE imm sys?
BCR (ie IgM, IgD)
TCR (MHC I & MCH II)
Antibody/Ab (IgM, G, A, D, F)
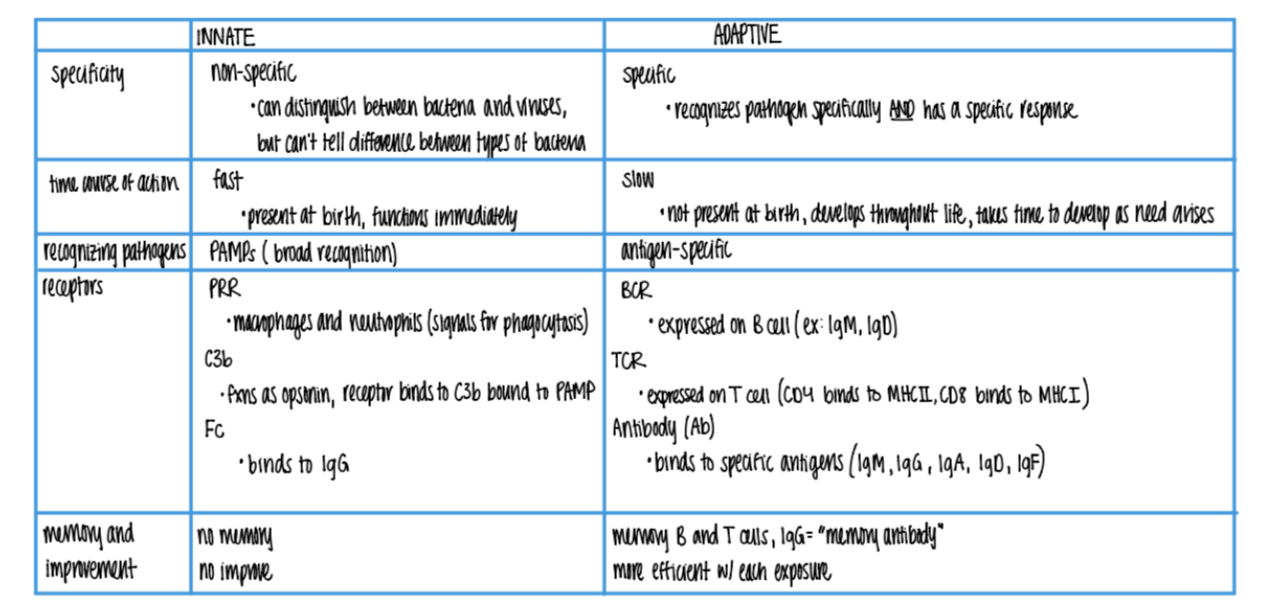
what is responsible for the “memory” in the adaptive imm sys?
B = makes IgG MEMORY antiBodies
T = quick activation via (CD4/CD8 APC) clonal selection
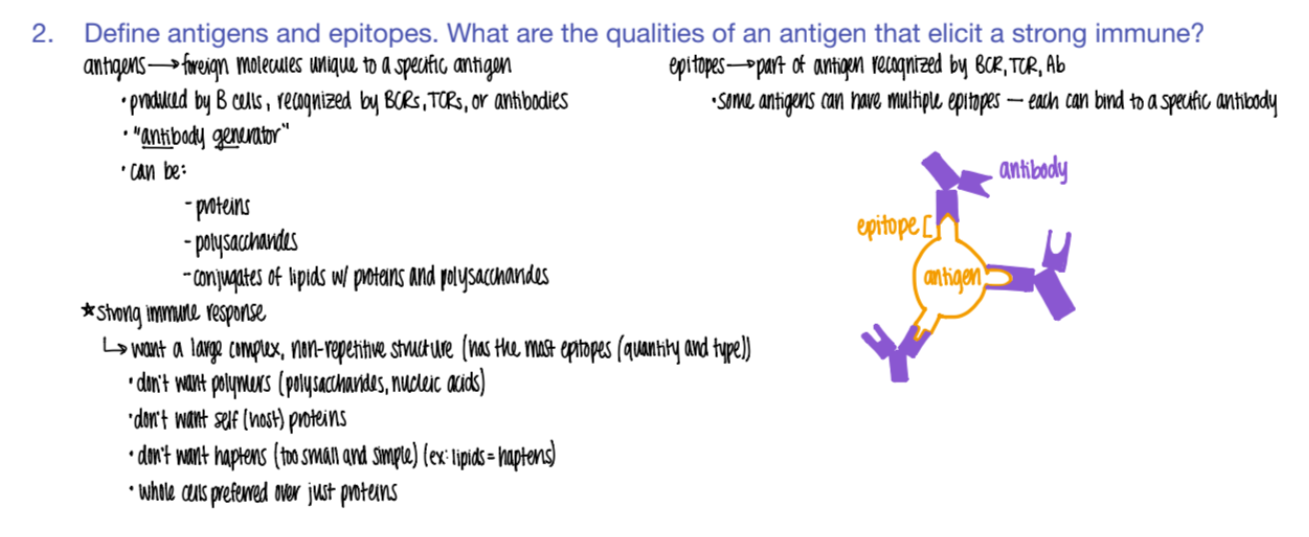
what types of mc’s can be antigens?
proteins
polysacc
lipids w/ proteins + polysacc!