Cytoskeletal Motor Proteins: Myosins, Kinesins, and Dyneins
0.0(0)
Card Sorting
1/21
Earn XP
Description and Tags
Last updated 1:00 AM on 3/25/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
1
New cards
Myosin motor proteins
\
* move along actin filaments by coupling the energy from ATP hydrolysis to conformational changes. They are considered mechanochemical enzymes (because they convert chemical to mechanical energy).
* The Myosin superfamily consists of at least 15 classes (I-XV) based on sequence homology in the head (motor) domains.
* Most Myosins are (+) end directed motors. - motor head domain
* Tails are doing different things but move using head domin
* Can dimerize them
* Myosin 6 moves towards (-) end
* move along actin filaments by coupling the energy from ATP hydrolysis to conformational changes. They are considered mechanochemical enzymes (because they convert chemical to mechanical energy).
* The Myosin superfamily consists of at least 15 classes (I-XV) based on sequence homology in the head (motor) domains.
* Most Myosins are (+) end directed motors. - motor head domain
* Tails are doing different things but move using head domin
* Can dimerize them
* Myosin 6 moves towards (-) end
2
New cards
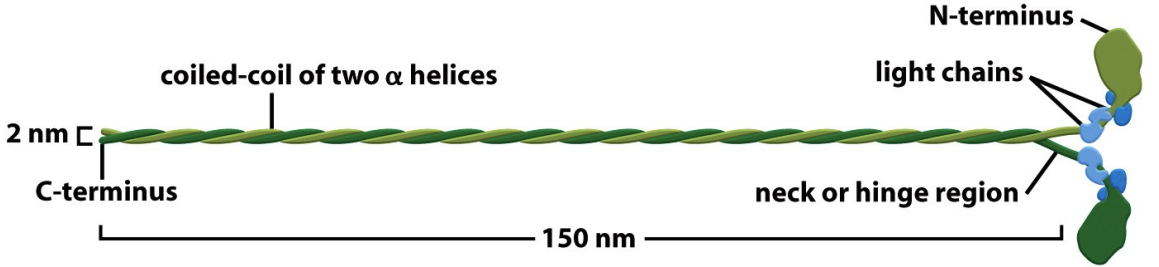
Myosins are composed of heavy & light chains
\
* The heavy chains have head, neck, and tail domains.
* The head (motor) domain contains the actin binding and the ATPase activities.
* The neck domain has a regulatory function and is the site of attachment for the regulatory and essential light chains or calmodulin.
* The tail domains differ between Myosins and determine specific properties of each one: what it will bind to, whether it will be a monomer or dimer, and whether it will polymerize into thick filaments.
* Myosin light chains bind the neck domain of the heavy chains and play structural and regulatory roles to control movement
* The heavy chains have head, neck, and tail domains.
* The head (motor) domain contains the actin binding and the ATPase activities.
* The neck domain has a regulatory function and is the site of attachment for the regulatory and essential light chains or calmodulin.
* The tail domains differ between Myosins and determine specific properties of each one: what it will bind to, whether it will be a monomer or dimer, and whether it will polymerize into thick filaments.
* Myosin light chains bind the neck domain of the heavy chains and play structural and regulatory roles to control movement
3
New cards
Myosin II
\
* was first described as the motor protein in muscle that powers contraction. Other Myosin II isoforms are also found in non-muscle cells.
* It consists of two heavy chains (\~200kD), each with an essential and a regulatory light chain (each 20kD; 6 proteins total).
* The tails are long α-helices that mediate dimerization by forming a coiled coil structure.
* The tails also mediate the polymerization of Myosin II into bipolar thick filaments.
* was first described as the motor protein in muscle that powers contraction. Other Myosin II isoforms are also found in non-muscle cells.
* It consists of two heavy chains (\~200kD), each with an essential and a regulatory light chain (each 20kD; 6 proteins total).
* The tails are long α-helices that mediate dimerization by forming a coiled coil structure.
* The tails also mediate the polymerization of Myosin II into bipolar thick filaments.
4
New cards
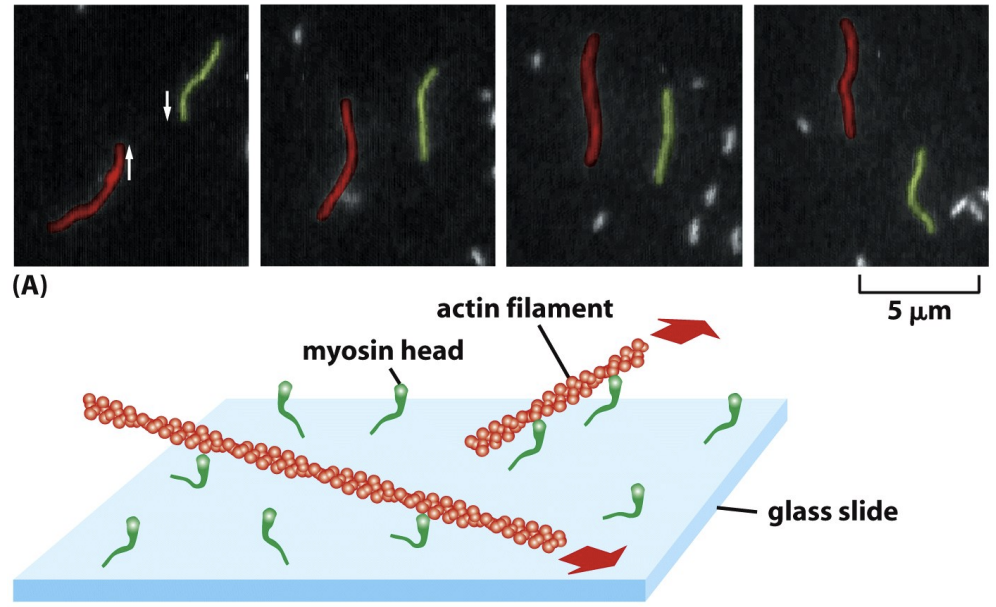
Actin filament sliding assay
\
* The motor function of Myosins can be directly demonstrated by video microscopy of filament sliding assays.
* In these experiments, myosin molecules are stuck to glass such that some bind by their tails with their head domains free. If fluorescent actin filaments are added without ATP (labeled with phalloidin), the filaments stick to the Myosin on the glass. If ATP is added, the filaments slide along the glass because actin is moving. The velocity of movement differs among distinct Myosins.
* Movement is ATP dependent
* ATP hydrolysis is coupled to Myosin motility, and Myosin ATPase activity is actin-activated. In the absence of actin the rate of hydrolysis is 4 ATP/hour, whereas in the presence of actin it is 20 ATP/second. This ensures that Myosin is active and using ATP only when actin is around.
* Which are the (+) ends? Which are the (–) ends? Is the + or – end leading? (-) leading because motors move towards (+) so driving (-) to lead movement
* The motor function of Myosins can be directly demonstrated by video microscopy of filament sliding assays.
* In these experiments, myosin molecules are stuck to glass such that some bind by their tails with their head domains free. If fluorescent actin filaments are added without ATP (labeled with phalloidin), the filaments stick to the Myosin on the glass. If ATP is added, the filaments slide along the glass because actin is moving. The velocity of movement differs among distinct Myosins.
* Movement is ATP dependent
* ATP hydrolysis is coupled to Myosin motility, and Myosin ATPase activity is actin-activated. In the absence of actin the rate of hydrolysis is 4 ATP/hour, whereas in the presence of actin it is 20 ATP/second. This ensures that Myosin is active and using ATP only when actin is around.
* Which are the (+) ends? Which are the (–) ends? Is the + or – end leading? (-) leading because motors move towards (+) so driving (-) to lead movement
5
New cards
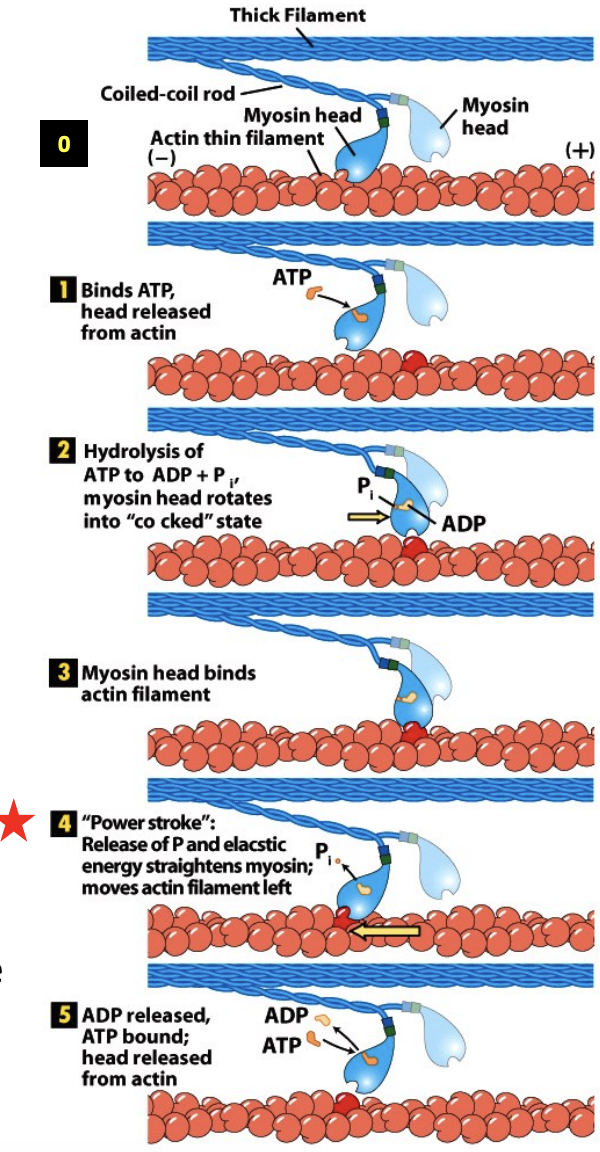
Myosin -Actin Cross -bridge Cycle
\
* Myosin II complex that is part of a thick filament, 2 heads,
* Myosin heads act independently, only one head does this process at a time
* Rigor state - at the start of the cycle, the ATP binding site is empty and Myosin head is tightly bound to actin. (0) Head is bent backwards like it is contracting. In muscle this state corresponds to rigor mortis – when muscle is depleted of ATP after death, the muscle becomes stiff because all of the Myosin is bound to actin.
* ATP binding – when ATP binds to Myosin, the ATP binding cleft closes, which opens the actin binding cleft and weakens the interaction with actin, lets go. (1)
* ATP hydrolysis – after detaching from the actin filament ATP is hydrolyzed to ADP and Pi. This causes a conformational change that moves the head to a new position that is more forward before rebinding the filament to ATP more towards the plus end.(2-3)
* Pi release – As the phosphate is released the Myosin head undergoes a second conformational change called the power stroke that restores Myosin to the rigor conformation. This exerts a force on the actin filament causing it to move relative to the Myosin. (4)
* ADP release – after ADP release the Myosin remains in the rigor state. ATP exchange releases the head from actin (5). The exact nature of each of these steps is still debated .
* Myosin II complex that is part of a thick filament, 2 heads,
* Myosin heads act independently, only one head does this process at a time
* Rigor state - at the start of the cycle, the ATP binding site is empty and Myosin head is tightly bound to actin. (0) Head is bent backwards like it is contracting. In muscle this state corresponds to rigor mortis – when muscle is depleted of ATP after death, the muscle becomes stiff because all of the Myosin is bound to actin.
* ATP binding – when ATP binds to Myosin, the ATP binding cleft closes, which opens the actin binding cleft and weakens the interaction with actin, lets go. (1)
* ATP hydrolysis – after detaching from the actin filament ATP is hydrolyzed to ADP and Pi. This causes a conformational change that moves the head to a new position that is more forward before rebinding the filament to ATP more towards the plus end.(2-3)
* Pi release – As the phosphate is released the Myosin head undergoes a second conformational change called the power stroke that restores Myosin to the rigor conformation. This exerts a force on the actin filament causing it to move relative to the Myosin. (4)
* ADP release – after ADP release the Myosin remains in the rigor state. ATP exchange releases the head from actin (5). The exact nature of each of these steps is still debated .
6
New cards
Mammalian cells express multiple Myosins
\
* Tail domains differ between different myosins and determine specific properties
* What will it bind to?
* Will it be a monomer or dimer?
* Will further polymerize into thick filaments?
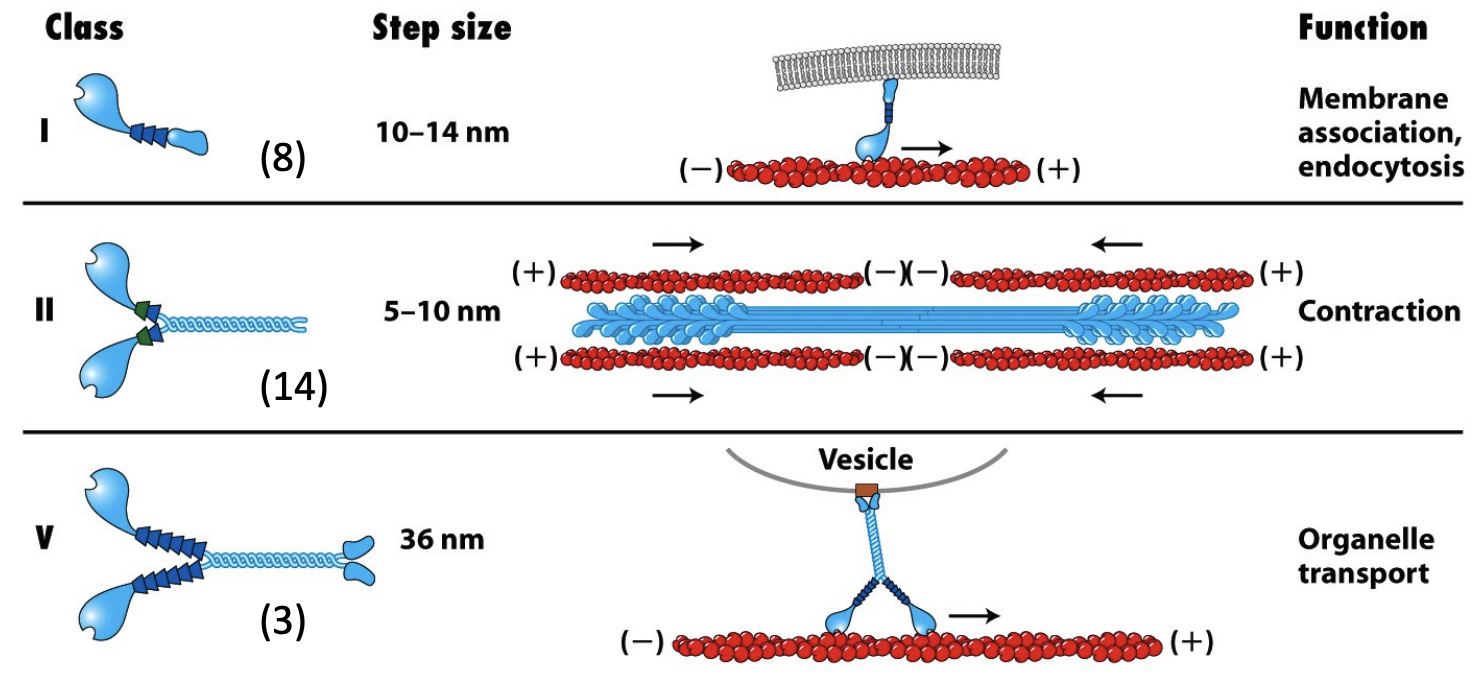
* Myosin I proteins have shorter tail domains and do not dimerize (they are single-headed and do not assemble into filaments).
* The tails of different Myosin I proteins are distinct. Some have a second actin binding site and mediate movement of filaments past one another. Some have membrane-binding sites that attach to and move vesicles or organelles.
* Myosin V proteins are dimers like Myosin II but are thought to be involved in vesicle transport like Myosin I.
* Myosin II can form thick filament
* Myosins III, IV and VI-XV have conserved head domains with variable tail regions. Their functions are not well characterized.
* Myosin VI is the only known Myosin to move towards the (–) end of F-actin
* Tail domains differ between different myosins and determine specific properties
* What will it bind to?
* Will it be a monomer or dimer?
* Will further polymerize into thick filaments?
* Myosin I proteins have shorter tail domains and do not dimerize (they are single-headed and do not assemble into filaments).
* The tails of different Myosin I proteins are distinct. Some have a second actin binding site and mediate movement of filaments past one another. Some have membrane-binding sites that attach to and move vesicles or organelles.
* Myosin V proteins are dimers like Myosin II but are thought to be involved in vesicle transport like Myosin I.
* Myosin II can form thick filament
* Myosins III, IV and VI-XV have conserved head domains with variable tail regions. Their functions are not well characterized.
* Myosin VI is the only known Myosin to move towards the (–) end of F-actin
7
New cards
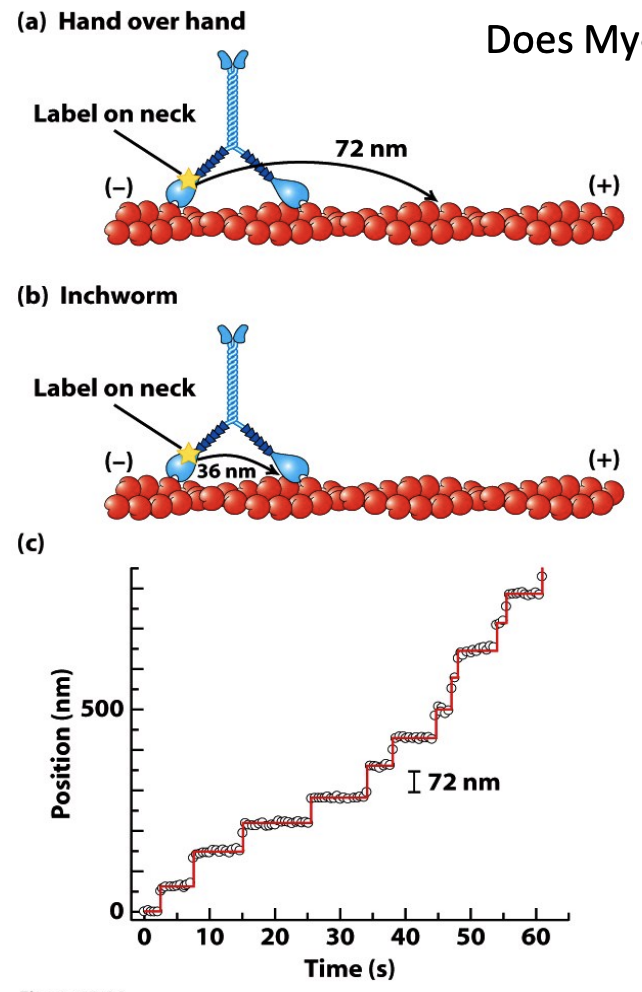
Myosin V dimers can ‘walk’ along F-actin
\
* Both heads work at the same time, one head always in contact so it does not fall off
* Does Myosin V walk hand-over-hand or like an inchworm?
* Experiment to find out:
* Fluorescently label the head and see how it moves - Hand over hand would move 72, inchworm would go 36
* The step size of a Myosin V motor walking along a single actin filament is 72nm. - hand over hand model
* If the cargo was labeled (instead of the neck), how far would it move per step?
* Unlike myosin II, the the binding and release of the two myosin V heads are coordinated, with one head always in contact with the actin. This processive movement allows it to reliably carry cargo.
* Both heads work at the same time, one head always in contact so it does not fall off
* Does Myosin V walk hand-over-hand or like an inchworm?
* Experiment to find out:
* Fluorescently label the head and see how it moves - Hand over hand would move 72, inchworm would go 36
* The step size of a Myosin V motor walking along a single actin filament is 72nm. - hand over hand model
* If the cargo was labeled (instead of the neck), how far would it move per step?
* Unlike myosin II, the the binding and release of the two myosin V heads are coordinated, with one head always in contact with the actin. This processive movement allows it to reliably carry cargo.
8
New cards
\
Mutations in Myosin genes can cause disease
Mutations in Myosin genes can cause disease
\
* More than 45 mutations in the MYH9 gene (encoding non-muscle Myosin IIA) have been found to cause diseases called “MYH9-related disorders”. They are characterized by bleeding problems, hearing loss, kidney disease, and clouding of the front surface of the eye (cataracts). Mutations at Arg702 in the protein result in many problems, including a severely reduced amount of platelets (thrombocytopenia), early-onset renal disease, and hearing loss in infancy.
* Myosin VI ablation has been associated with deafness in mice.
* Myosin VII mutations have been associated with deafness and blindness in humans (Usher Syndrome 1B, autosomal dominant non-syndromic deafness).
* More than 45 mutations in the MYH9 gene (encoding non-muscle Myosin IIA) have been found to cause diseases called “MYH9-related disorders”. They are characterized by bleeding problems, hearing loss, kidney disease, and clouding of the front surface of the eye (cataracts). Mutations at Arg702 in the protein result in many problems, including a severely reduced amount of platelets (thrombocytopenia), early-onset renal disease, and hearing loss in infancy.
* Myosin VI ablation has been associated with deafness in mice.
* Myosin VII mutations have been associated with deafness and blindness in humans (Usher Syndrome 1B, autosomal dominant non-syndromic deafness).
9
New cards
Kinesins and Dyneins intro
\
* Advances in video microscopy in the 1970s and 1980s facilitated the observation of organelles and particles moving along single MTs. This phenomenon could also be observed when cell cytoplasm (from the axons of giant neurons in squid) was added to MTs in the presence of ATP. These in vitro motility assays were used to isolate two classes of MT motor proteins – Kinesins and Dyneins.
* Kinesins and Myosins have remarkably similar ATP binding/hydrolysis catalytic core domains. This appears to have been convergent evolution, as opposed to divergence from a common ancestor protein.
* Dynein’s catalytic core is unique
* Advances in video microscopy in the 1970s and 1980s facilitated the observation of organelles and particles moving along single MTs. This phenomenon could also be observed when cell cytoplasm (from the axons of giant neurons in squid) was added to MTs in the presence of ATP. These in vitro motility assays were used to isolate two classes of MT motor proteins – Kinesins and Dyneins.
* Kinesins and Myosins have remarkably similar ATP binding/hydrolysis catalytic core domains. This appears to have been convergent evolution, as opposed to divergence from a common ancestor protein.
* Dynein’s catalytic core is unique
10
New cards
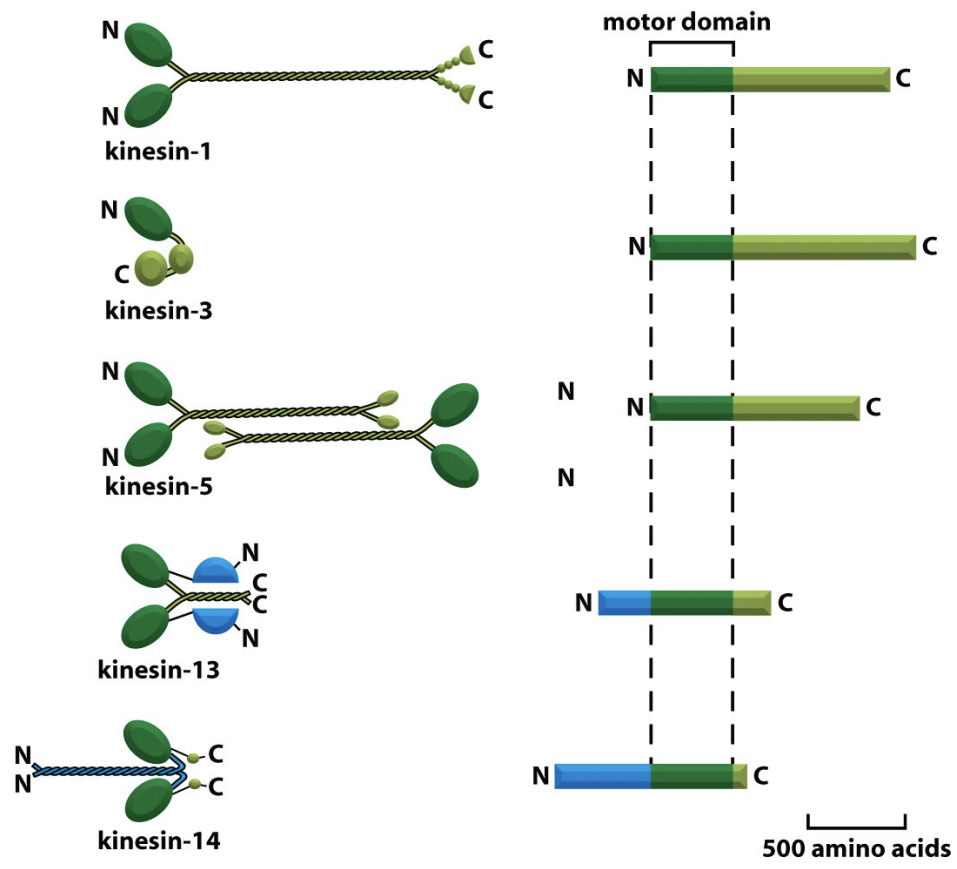
Kinesins
\
* have conserved motor domains
* Kinesins are MT-activated mechanochemical ATPases. They comprise a large superfamily of related proteins that fall into 3 major classes. Each of these classes shares a high degree of sequence similarity in the motor domain, which contains the ATPase and MT binding activity.
* The 3 classes differ in the location of the motor domain in the primary sequence of the protein:
* Kin-N Kinesins
* Kin-C Kinesins
* Kin-I Kinesins
* have conserved motor domains
* Kinesins are MT-activated mechanochemical ATPases. They comprise a large superfamily of related proteins that fall into 3 major classes. Each of these classes shares a high degree of sequence similarity in the motor domain, which contains the ATPase and MT binding activity.
* The 3 classes differ in the location of the motor domain in the primary sequence of the protein:
* Kin-N Kinesins
* Kin-C Kinesins
* Kin-I Kinesins
11
New cards
Kin-N Kinesins
\
* have the motor domain at the N-terminus of the protein. Kin-N Kinesins (including conventional Kinesin) move towards the MT (+) ends.
* have the motor domain at the N-terminus of the protein. Kin-N Kinesins (including conventional Kinesin) move towards the MT (+) ends.
12
New cards
Kin-C Kinesins
\
* have a C-terminal motor domain. Kin-C Kinesins move towards the (-) ends (these Kinesins are rare).
* have a C-terminal motor domain. Kin-C Kinesins move towards the (-) ends (these Kinesins are rare).
13
New cards
Kin-I Kinesins
\
* have an internal motor domain (centrally located in the protein sequence). Kin-I Kinesins do not move along MTs, but bind MT ends and promote protofilament peeling. - Kin13 - depolarize ends
* have an internal motor domain (centrally located in the protein sequence). Kin-I Kinesins do not move along MTs, but bind MT ends and promote protofilament peeling. - Kin13 - depolarize ends
14
New cards
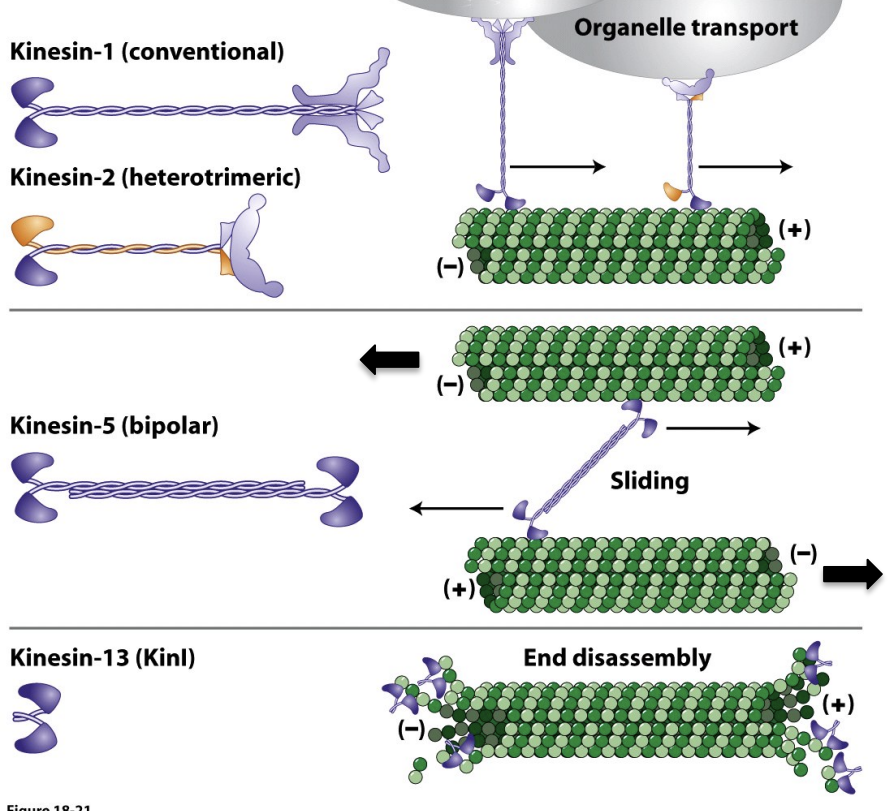
Kinesins differ in organization and function
\
* Conventional Kinesin was the first Kinesin discovered and is the best characterized.
* It consists of two heavy chains and two light chains. The motor domain head is located at the N-terminus of the heavy chain and is followed by the neck domain and the α-helical coiled-coil tail (stalk) domain.
* Unlike the non-muscle Myosin II light chains, Kinesin light chains mediate interaction of Kinesin with membrane vesicles.
* 2 kinesin 5 can interact and form a bipolar structure, this causes microtubule sliding, microtubules are antiparallel, slide in opposite directions
* Kinesin 13 not used for walking, drives depolymerization of microtubules
* Conventional Kinesin was the first Kinesin discovered and is the best characterized.
* It consists of two heavy chains and two light chains. The motor domain head is located at the N-terminus of the heavy chain and is followed by the neck domain and the α-helical coiled-coil tail (stalk) domain.
* Unlike the non-muscle Myosin II light chains, Kinesin light chains mediate interaction of Kinesin with membrane vesicles.
* 2 kinesin 5 can interact and form a bipolar structure, this causes microtubule sliding, microtubules are antiparallel, slide in opposite directions
* Kinesin 13 not used for walking, drives depolymerization of microtubules
15
New cards
Microtubule gliding assay
\
* The motor function of Kinesin can be directly demonstrated by microscopic gliding assays. These experiments resemble those shown with Myosin molecules earlier. (In which direction are the MTs moving?)
* Kinesins on coverslips with heads, tails exposed, put microtubules
* You will see microtubules being moved around, (-) ends leading moving to (+) end
* C-kinesins will be (+) leading
* The motor function of Kinesin can be directly demonstrated by microscopic gliding assays. These experiments resemble those shown with Myosin molecules earlier. (In which direction are the MTs moving?)
* Kinesins on coverslips with heads, tails exposed, put microtubules
* You will see microtubules being moved around, (-) ends leading moving to (+) end
* C-kinesins will be (+) leading
16
New cards
Kinesin Cross-bridge Cycle
\
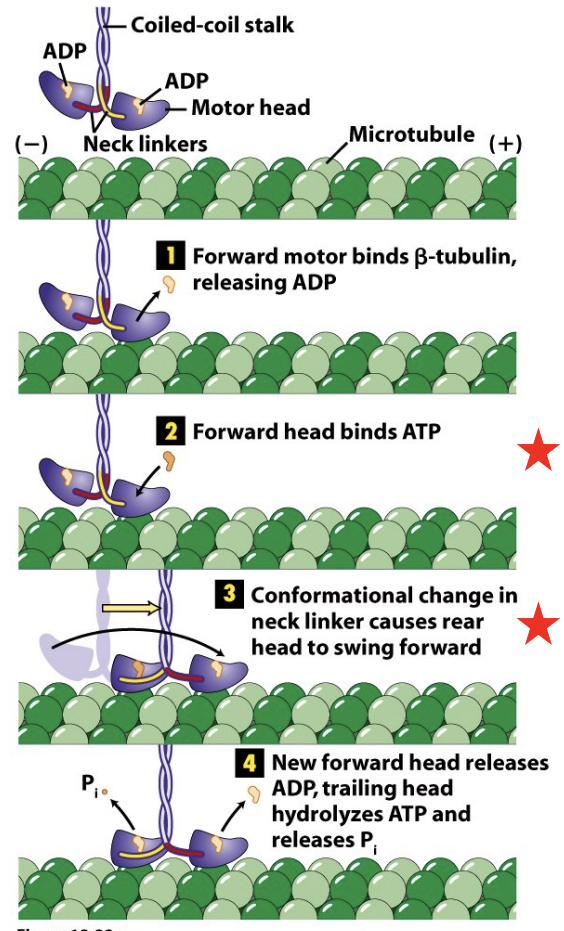
* Kinesins undergo a cross-bridge cycle similar to that of Myosins in which ATP hydrolysis and Pi release are coupled to conformational changes in the head and neck domains of the molecule
* A key step is when ATP binds to the forward head, causing changes, rear head swings forward past front head to have a forward position
* Kinesins undergo a cross-bridge cycle similar to that of Myosins in which ATP hydrolysis and Pi release are coupled to conformational changes in the head and neck domains of the molecule
* A key step is when ATP binds to the forward head, causing changes, rear head swings forward past front head to have a forward position
17
New cards
Kinesin motor proteins can move processively
\
* In its ATP-driven movement along a MT, Kinesin-1 can take more than 100 steps without falling off the MT. This behavior – movement over long distances without dissociating – is termed processive motility (analogous to the processive polymerase activity of RNA and DNA polymerases). Processivity is important for long-distance transport of vesicles and organelles.
* Processive movement - use both heads
* In its ATP-driven movement along a MT, Kinesin-1 can take more than 100 steps without falling off the MT. This behavior – movement over long distances without dissociating – is termed processive motility (analogous to the processive polymerase activity of RNA and DNA polymerases). Processivity is important for long-distance transport of vesicles and organelles.
* Processive movement - use both heads
18
New cards
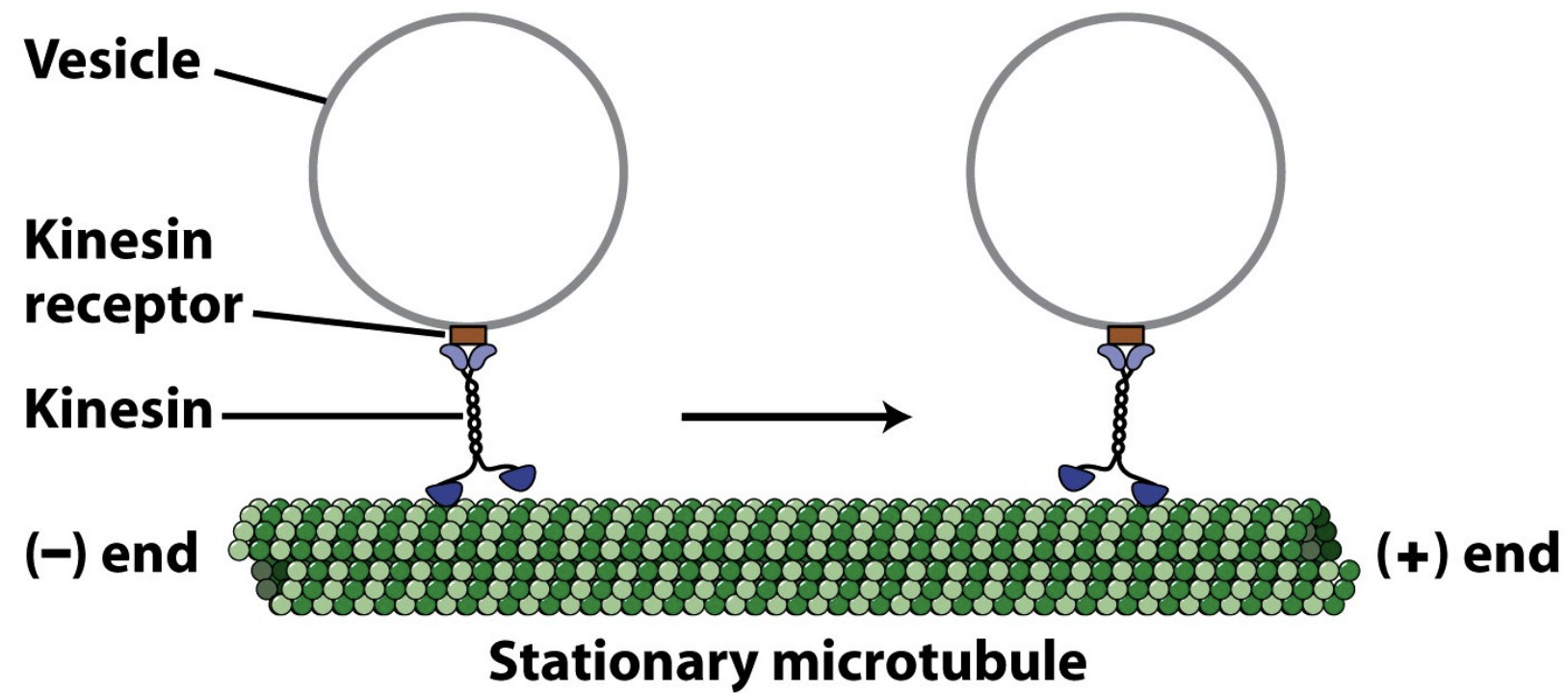
Processive movements of Kinesin motor proteins are important for vesicle transport
\
* Trafficking using kinesin is useful
* Kinesin walking towards (+) end carrying vesicles
* Trafficking using kinesin is useful
* Kinesin walking towards (+) end carrying vesicles
19
New cards
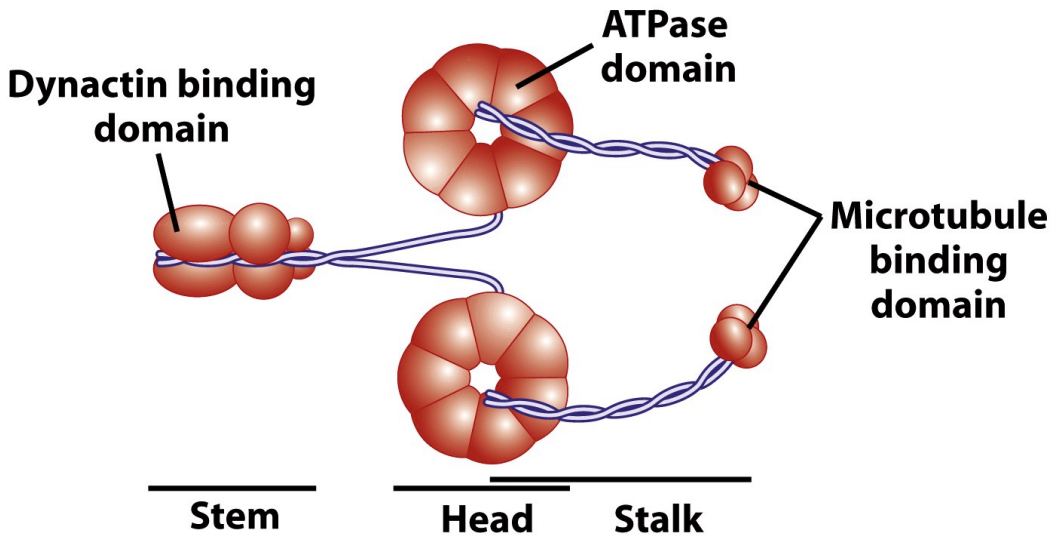
Dyneins
* have conserved motor domains
* Dyneins are mechanochemical enzymes that couple the energy from ATP hydrolysis to (–) end directed movement along MTs. Dyneins are huge (greater than 1,000 kDa) motor molecules composed of 2-3 heavy chains (about 450 kDa each) complexed with a poorly characterized number of intermediate and light chains.
* Microtubule binding domain binds to microtubule
* ATPase domain binds to ATP causing conformational changes
* Dynactin binding domain binds to cargo
There are two major types of dynein: Cytoplasmic Dynein (below) is the most common form important for many cellular functions. The closely-related Ciliary/Flagellar Dynein is involved in ciliary and flagellar motility (more later).
\
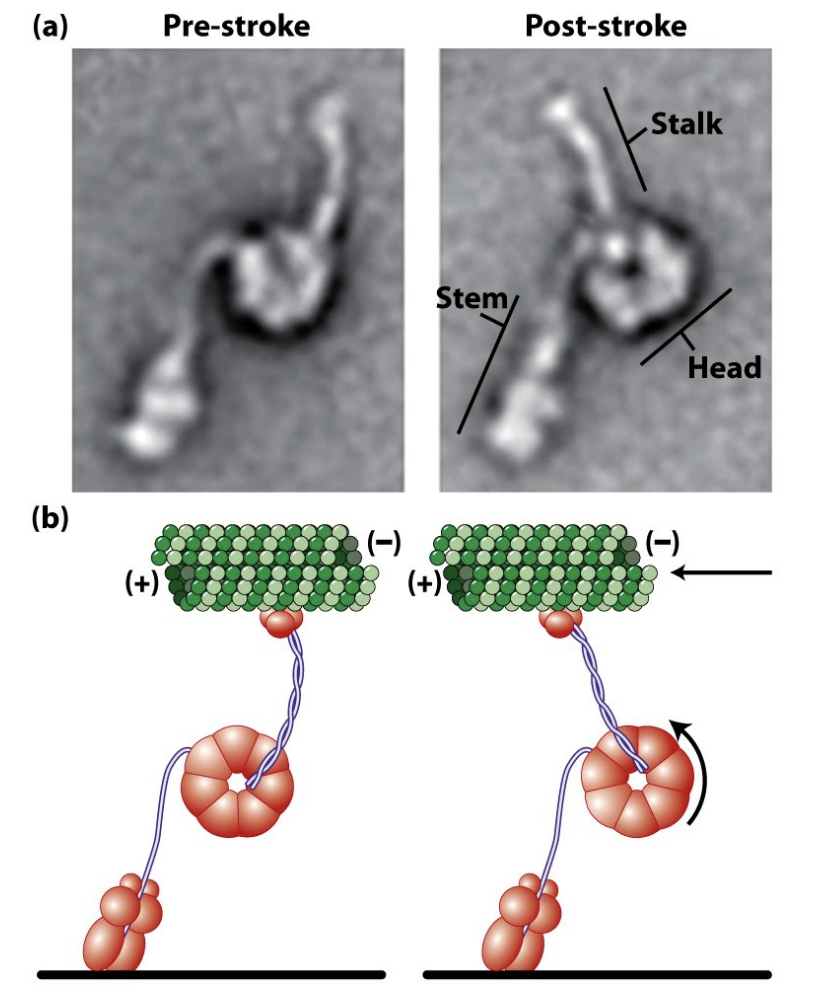
* Dynein has a power stroke too
* Dyneins are mechanochemical enzymes that couple the energy from ATP hydrolysis to (–) end directed movement along MTs. Dyneins are huge (greater than 1,000 kDa) motor molecules composed of 2-3 heavy chains (about 450 kDa each) complexed with a poorly characterized number of intermediate and light chains.
* Microtubule binding domain binds to microtubule
* ATPase domain binds to ATP causing conformational changes
* Dynactin binding domain binds to cargo
There are two major types of dynein: Cytoplasmic Dynein (below) is the most common form important for many cellular functions. The closely-related Ciliary/Flagellar Dynein is involved in ciliary and flagellar motility (more later).
\
* Dynein has a power stroke too
20
New cards
Dynactin complex
\
* The Dynactin complex links Dynein to cargo
* The Dynactin complex is a multisubunit protein complex that is critical for the function of cytoplasmic Dynein. It co-purifies with Dynein and is thought to mediate the attachment of Dynein to vesicles and organelles. The Dynactin complex consists of \~11 proteins.
* Cargo attaches to complex which then attaches to binding domain
* Arp1(help nucleate microfilament growth) and CapZ (cap polymer)
\
* The dynactin complex may use its Actin Related Protein 1 (Arp1) filament to interact with Spectrin/Ankyrin membrane complexes on the surface of the vesicle cargo.
* The Dynactin complex links Dynein to cargo
* The Dynactin complex is a multisubunit protein complex that is critical for the function of cytoplasmic Dynein. It co-purifies with Dynein and is thought to mediate the attachment of Dynein to vesicles and organelles. The Dynactin complex consists of \~11 proteins.
* Cargo attaches to complex which then attaches to binding domain
* Arp1(help nucleate microfilament growth) and CapZ (cap polymer)
\
* The dynactin complex may use its Actin Related Protein 1 (Arp1) filament to interact with Spectrin/Ankyrin membrane complexes on the surface of the vesicle cargo.
21
New cards
Microtubule motors are implicated in disease
\
* Kinesin deficiencies are associated with Charcot-Marie-Tooth disease and some kidney diseases.
* Dynein deficiencies can lead to chronic infections of the respiratory tract, because cilia fail to function without dynein.
* Mutations in the dynactin complex ( p150Glued subunit) have been linked to both familial and sporadic ALS
* Defects in ciliary and intraflagellar transport cause autosomal recessive polycystic kidney disease (ARPKD), retinal degeneration, and several other sensory disorders
* Kinesin deficiencies are associated with Charcot-Marie-Tooth disease and some kidney diseases.
* Dynein deficiencies can lead to chronic infections of the respiratory tract, because cilia fail to function without dynein.
* Mutations in the dynactin complex ( p150Glued subunit) have been linked to both familial and sporadic ALS
* Defects in ciliary and intraflagellar transport cause autosomal recessive polycystic kidney disease (ARPKD), retinal degeneration, and several other sensory disorders
22
New cards
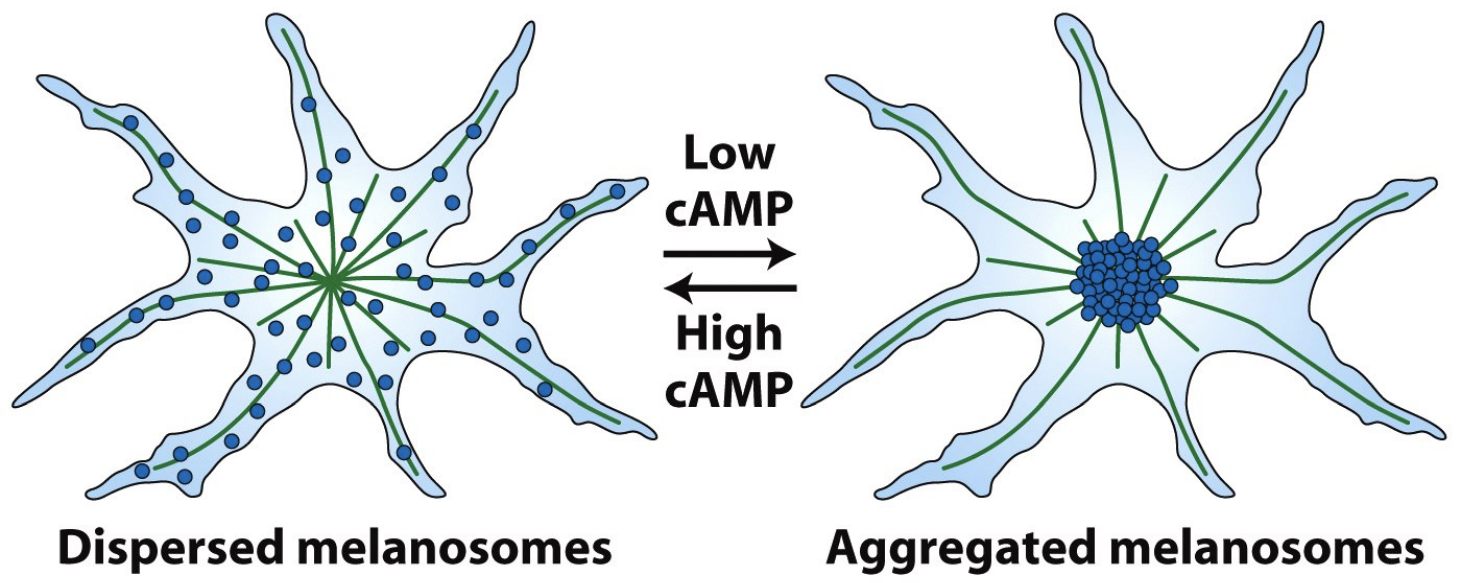
Questions to ponder
\
* Where are the microtubule (+) ends and (-) ends ?
* - by the centrosome, + are outward
* Are Kinesins or Dyneins important for melanosome localization in high cAMP ?
* kinesins
* Are Kinesins or Dyneins important for melanosome localization in low cAMP ?
* Dyneins
* What will melanosome organization look like if you treat cells with taxol ?
* Move just fine
* What will melanosome organization look like if you treat cells with nocodazole ?
* No melanosome organization
* What happens when you wash away the nozodazole or taxol ?
* Regrowth from centrosome and grow out and are normal
* Where are the microtubule (+) ends and (-) ends ?
* - by the centrosome, + are outward
* Are Kinesins or Dyneins important for melanosome localization in high cAMP ?
* kinesins
* Are Kinesins or Dyneins important for melanosome localization in low cAMP ?
* Dyneins
* What will melanosome organization look like if you treat cells with taxol ?
* Move just fine
* What will melanosome organization look like if you treat cells with nocodazole ?
* No melanosome organization
* What happens when you wash away the nozodazole or taxol ?
* Regrowth from centrosome and grow out and are normal