Atomic Theory Unit Flashcards
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
what was wrong with the nuclear model of the atom/rutherford’s model?
electron is vibrating, circling around the nucleus
radiating light and radiation, therefore losing energy
if energy is being lost, it should be slowing down and falling into the nucleus
since it does not fall into the nucleus, there is a flaw in the model
what explained the problem of “collapsing” atoms
the bohr model of the atom
3 postulates of bohr model of atom
electrons in atoms may only occupy specific energy states called stationary states, each corresponds to a well defined circular orbit around the nucleus → energy is quantized: only has specific certain values
electrons in stationary states do not emit energy
electrons can change stationary states by absorbing or emitting a quantum of energy exactly equal to the energy difference between the two states
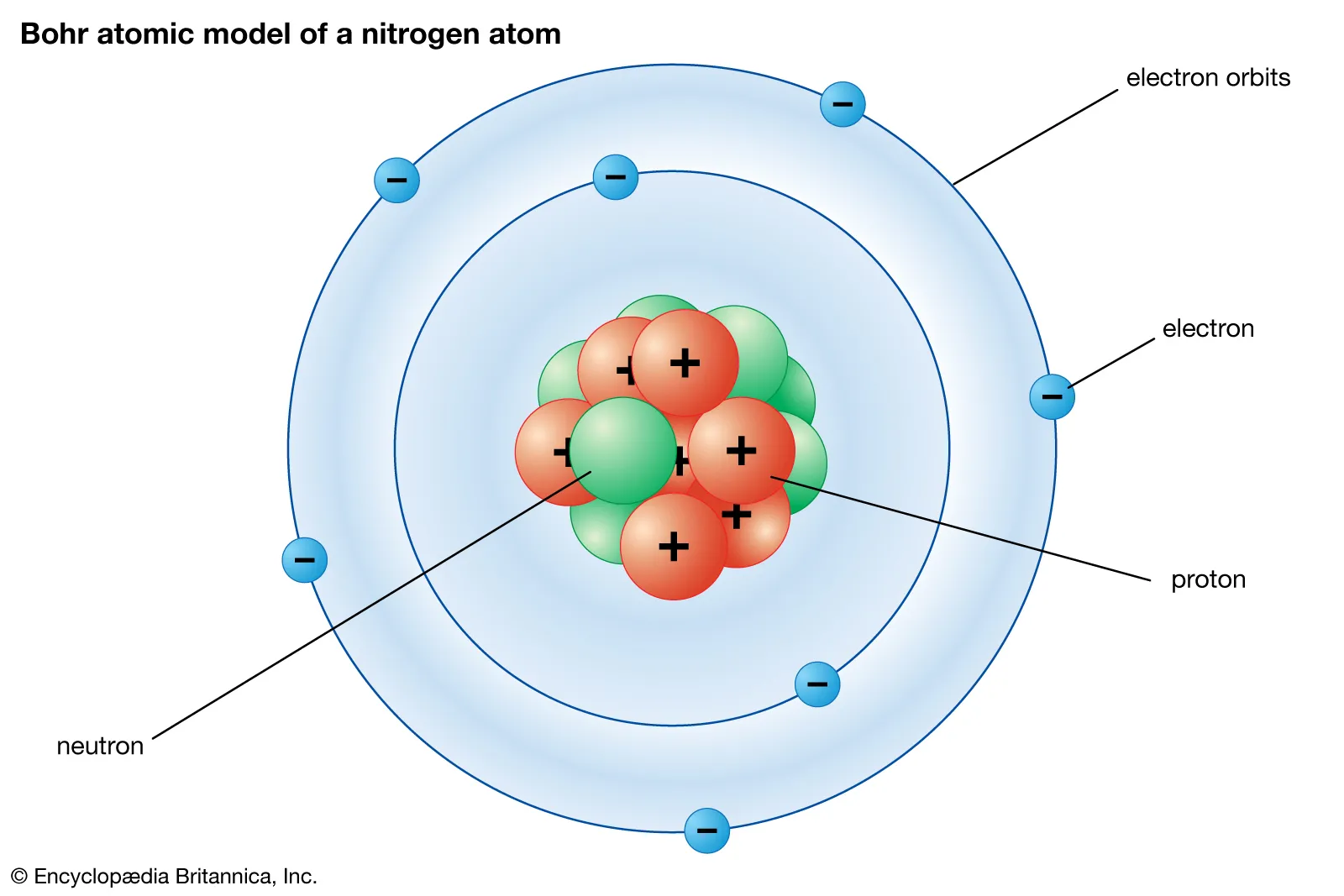
ground state
lowest energy arrangement of electron
closest state electron can be to proton
excited state
NOT the lowest energy state
not lowest electron arrangement in atom
any other level besides ground
infinite amount
electrostatic force
attraction or repulsion of electrons and protons
quantized and quantum
Quantized: has a certain specific value
Quantum: Something that has a certain specific value
when does an electron have greater potential energy?
greater potential energy when in excited state, the further it gets from nucleus
why doesnt the electron fall into the nucleus?
the electron can come closer to ground state by releasing a quantum of energy
the electron can go further from the nucleus by absorbing a quantum of energy
it does not continuously move, can only absorb or release exact amount of energy and goes to next orbit without staying in between the orbits
the closest the electron can be to nucleus is in ground state
nucelons
particles found in the nucleus, in our case protons and neutrons
standard atomic symbol
A in top left corner: atomic mass number
Z in bottom left corner: atomic number
X: element
charge: depends on protons and electrons

Z
atomic number
number of protons in nucleus
defines identity of element
found in top right corner on table
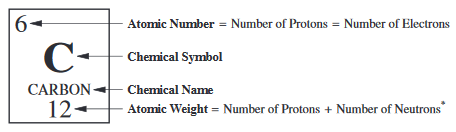
A
atomic mass number
total number of nucleons (protons and neutrons) in a nucleus
not the mass
will always be a whole number
found underneath the name of an element on table
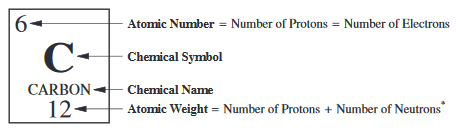
non standard atomic symbol
X - A, element dash mass number
how to determine number of protons, neutrons and electrons
#p: Z, atomic number
#n = Z - A, protons - electrons
#e = same as protons unless there is a charge
FIX THIS CARD
isotope
different forms of the same element that contain equal numbers of protons but different numbers of neutrons
differ in relative atomic mass but not in chemical properties
isotopes of hydrogen
protium: one p+, zero neutrons, one e-
deuterium: one p+, one neutron, one e-
tritium: one p+, two neutrons, one e-
radioisotope
unstable isotope that undergoes nuclear decay
ions
unequal number of protons and electrons
have a charge
electrons > protons: negatively charged anion
electron < protons: positively charged cation
allotrope
different forms of the same element that have different physical and chemical properties, i.e. charcoal and diamond are allotropes of carbon
electronic configuration
Arrangement of electrons around the nucleus in an atom
valence orbit/shell
Outermost orbit of electrons
inner orbit/shell
NOT the outermost orbit of electrons
principal quantum number (n)
use to determine total number of electrons depending on how many shells there are when shells are full
number of electrons = 2n2
i.e. in 3 shells, 2(3)2 = 18, the first orbit has 2, then 8, then 8 again
purpose of mass spectrometer
a sample is heated, vapourized and ionized
travels down to the detector
calculated force of the magnets that bend the sample
based on the magnetic force you know the masses and you know the relative amount of the
isotope
Each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element
how to determine average atomic mass?
Calculate average of the mass of the isotopes
Multiply masses by percentage
add them up
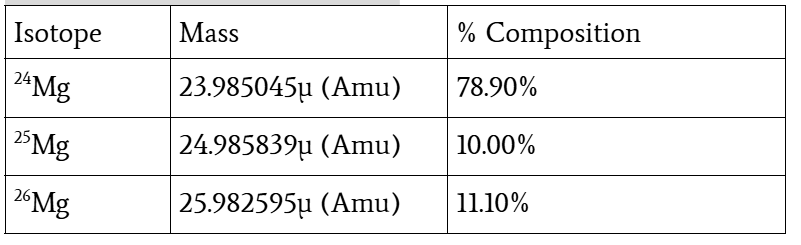
Determine the average atomic mass of a sample of magnesium based on the percent composition data in the picture
MAvg = (CMg-24 x MMg-24) + (CMg-25 x MMg-25) + (CMg-26 x MMg-26)
= (0.7890)(23.98504 amu) + (0.1000)(24.985839 amu) + (0.1110)(25.982595 amu)
= 24.306852 amu
= 24.31 amu
positioning of electrons
at 3
at 6
at 9
at 12
always fill first 4 positions before starting pairs
for Helium, draw the two as a pair (together) at the top or right
in the first orbit, the two electrons will always be a pair at the top or right
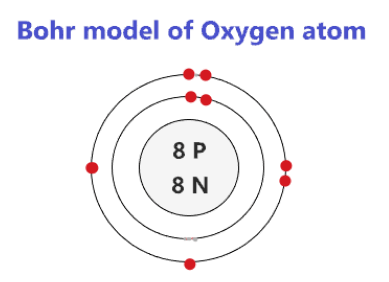
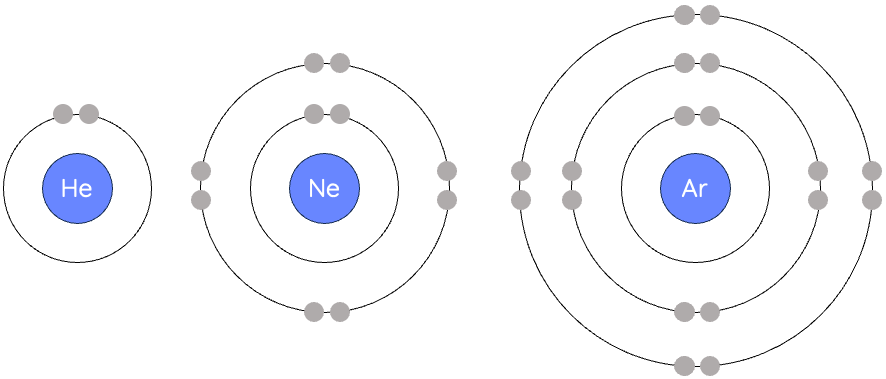
how to draw bohr rutherford diagram looking at periodic table
period # = # of shells
group # = # of valence electrons (groups 1-8)
first orbit : max 2 pairs
all other orbits: max 18 electrons, 4 pairs
nucleus: # P and # N written
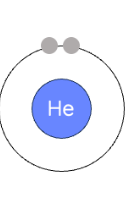
how to draw bohr diagram looking at periodic table
period # = # of shells
group # = # of valence electrons (groups 1-8)
first orbit : max 2 pairs
all other orbits: max 18 electrons, 4 pairs
nucelus: no cricle around it, just element symbol
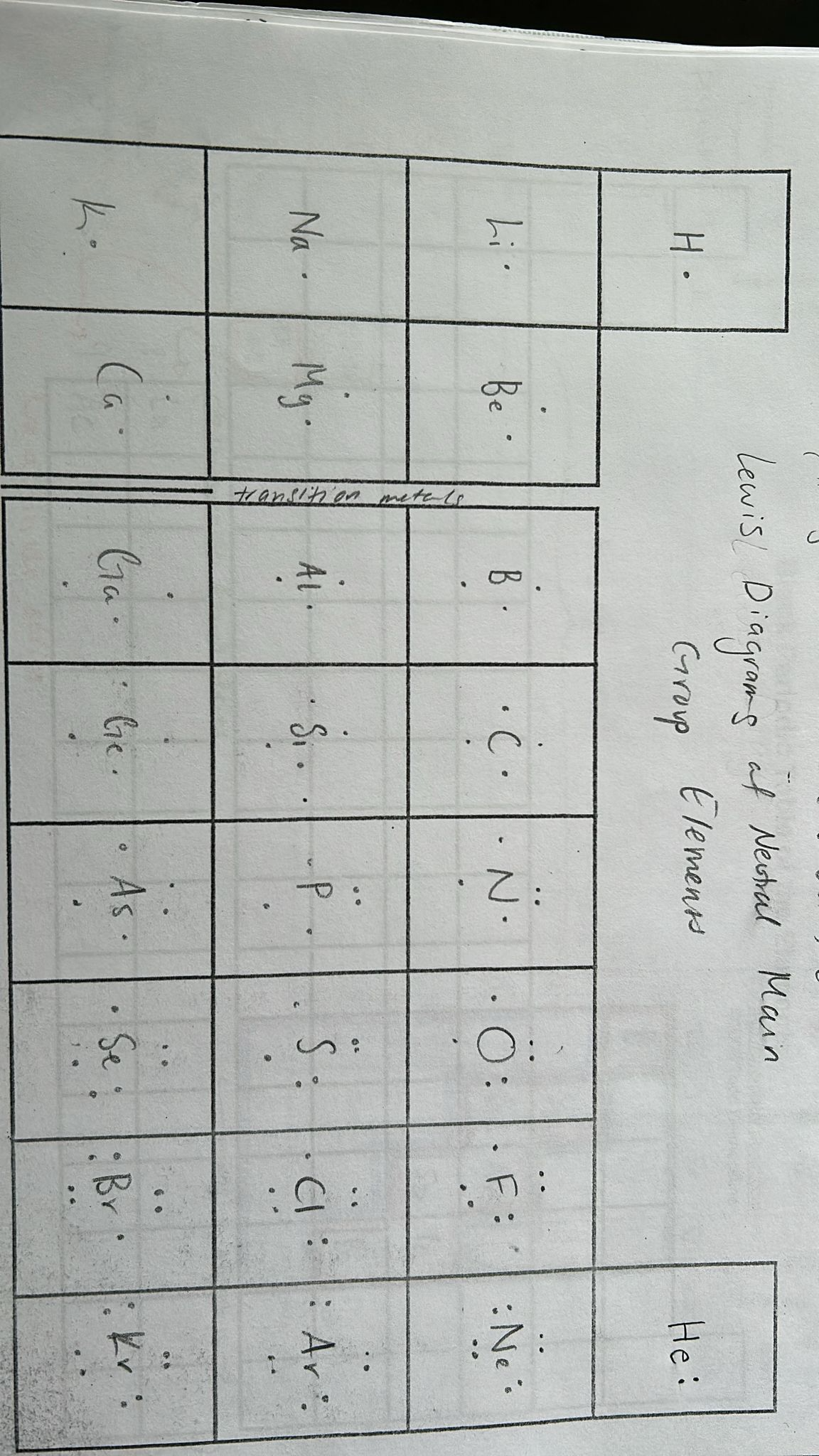
how to draw lewis diagrams
only includes valence electrons
He is the only one where e is in a pair on the right, all other elements have them separated (fill first 4 positions before starting pairs)
elements name in middle
summary of gold foil experiment
ernest rutherford
fired alpha particles (tiny and charged particles smaller than atom) at gold foil
found that 1 - majority of alpha α particles shoot straight through
2- a small fraction of alpha α particles found at small angles
3- tiny fraction of alpha α particles detected in front of the foil
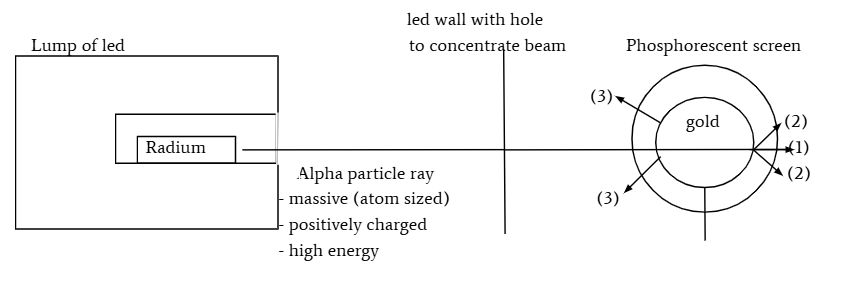
conclusions of gold foil experiment
most of atom is empty space
positively charged centers consist of majority of mass
mass and charged are not uniformly distributed
nuclear model of atom: positively charged nucleus with electrons orbiting nucleus
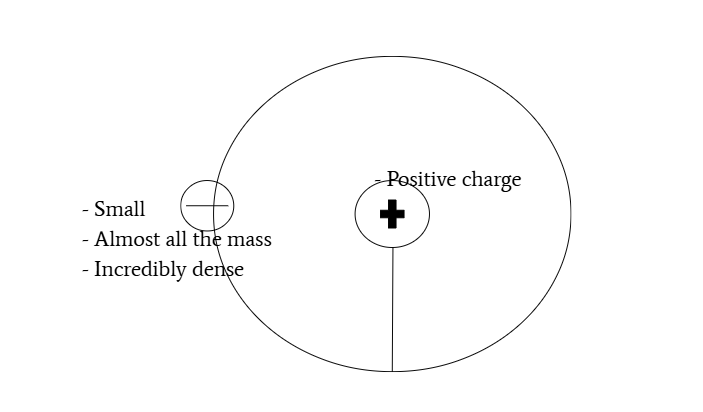
electrostatic force
Force of attraction and repulsion between protons and electrons
principal quantum number
orbits outside of nucleus
n = 1, 2, 3… infinity
electron is never between any two states
when electron goes closer/farther from nucleus
gives off a quantum of energy exactly equal to the difference between the two states when going from excited to ground
when going away from nucleus, ground to excited or further, absorb a quantum of energy exactly equal to the difference between the two states when going from excited to ground
what determines the attraction of electrons and protons
the attraction between electrons and protons depends on the number of protons
frequency is proportional to…
frequency is inversely proportional to wavelength
high frequency, short wavelength
low frequency, long wavelength
incandescent light is a
continuous spectrum
are orbits of electron dynamic or static?
dynamic
change according to forces/function of forces
different forces, different energy
line spectra and identifying elements
all elements give off a different spectra
spectras can be used to identify elements
the spectra depends on the energy in the element which depends on the force of attraction in the atom
electron is attracted to nucleus
the nucleus charge/number of protons determines the force of attraction
Every element has a different number of protons therefore different levels of attraction therefore different energy levels therefore different line spectrums
What is a key tenet of Dalton's Atomic Theory regarding elements?
Each type of atom makes up a specific substance we call an element.
According to Dalton's Atomic Theory, what happens to atoms in chemical reactions?
Atoms are never created or destroyed in chemical reactions.
How do atoms combine to form compounds, according to Dalton's Atomic Theory?
Atoms combine in simple whole number ratios to form compounds which are constant and define a particular substance.
What does measuring mass tell us about particles according to Dalton's ideas?
Measuring mass indicates the amount or number of particles, because particles (atoms) have mass.
What did William Crookes invent in the 1870s that contributed to understanding atoms?
He invented the Crookes tube.
What observation made within the Crookes tube suggested that cathode rays had a charge?
Magnets could change the polarity and move the rays.
What was J.J. Thomson's conclusion about the green glow in his Maltese cross experiment?
The green glow flows from the cathode to the anode and are negatively charged cathode rays.
What did the paddle wheel experiment demonstrate about cathode rays?
Cathode rays have mass and are made of particles because they can push the paddle wheel away from the cathode.
What did J.J. Thomson discover through quantitative experiments with cathode rays?
Cathode rays are much lighter than hydrogen atoms, and discovered electrons as the first subatomic particles.
Why did scientists believe there had to be positive charges within an atom?
Since electrons are negatively charged and matter is usually neutral, there has to be positively charged subatomic particles to balance the negative charge.
Describe the Plum Pudding Model of the atom.
JJ Thomposon: A ‘dough’ of positive charge with negatively charged electrons dispersed throughout it, with uniform mass and charge density, no concentrated areas
summary of crookes tube experiment
evacuated tube with a cathode (-) and anode (+) connected to a power supply emitted a green glow that could be deflected by magnets → it must consist of charge
summary of maltese cross experiment
a different shape of evacuated tube had a cathode at the tope and anode at the bottom, and a maltese cross at one end, when connected to a power supply emitted a green glow that created a shadow, and if polarity were switched no glow would appear → the glow travels from the cathode to anode, so they are negatively charged cathode rays
summary of paddle wheel experiment
(thompson or crookes) a paddle wheel inserted in an evacuated tube and the cathode rays caused the wheel to move → cathode rays have mass