FAMILIES & FUNCTIONAL GROUPS STRUCTURES
1/67
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
What is the functional group structure of an aromatic compound?
A six-carbon ring with alternating double bonds (benzene ring).
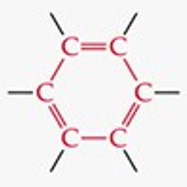
Give a simple example of an aromatic compound.
Benzene (C₆H₆).
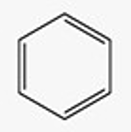
Recognize this line structure:
Aromatic compound.
What suffix is used for aromatics?
None.
What is the functional group structure of an alkane?
Contains only C–C and C–H single bonds; no readily reactive bonds.
Give a simple example of an alkane.
Propane (CH₃CH₂CH₃).
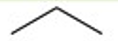
Recognize this line structure:
Alkane.
What is the suffix for alkanes?
-ane.
What is the functional group structure of an alkene?
Carbon–carbon double bond (C=C).
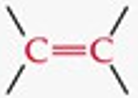
Give a simple example of an alkene.
Ethylene (H₂C=CH₂).
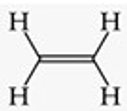
Recognize this line structure:
Alkene.
What is the suffix for alkenes?
-ene.
What is the functional group structure of an alkyne?
Carbon–carbon triple bond (C≡C).
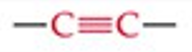
Give a simple example of an alkyne.
Acetylene (ethyne, HC≡CH).

Recognize this line structure:
Alkyne.
What is the suffix for alkynes?
-yne.
What is the functional group structure of an alkyl halide?
A carbon bonded to a halogen (F, Cl, Br, or I).
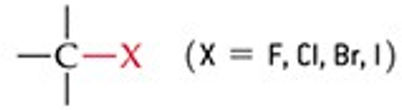
Give a simple example of an alkyl halide.
Ethyl chloride (CH₃CH₂Cl).
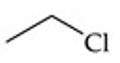
Recognize this line structure:
Alkyl halide.
What is the suffix for alkyl halides?
None.
What is the functional group structure of an alcohol?
Hydroxyl group (–OH).
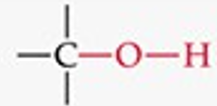
Give a simple example of an alcohol.
Ethanol (CH₃CH₂OH).
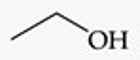
Recognize this line structure:
Alcohol.
What is the suffix for alcohols?
-ol.
What is the functional group structure of an ether?
Oxygen atom bonded to two carbons (R–O–R).
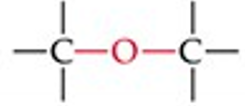
Give a simple example of an ether.
Diethyl ether (CH₃CH₂–O–CH₂CH₃).
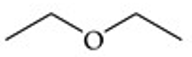
Recognize this line structure:
Ether.
What is the suffix for ethers?
None.
What is the functional group structure of an amine?
–NH₂ (amino group).
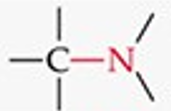
Give a simple example of an amine.
Ethylamine (CH₃CH₂NH₂).
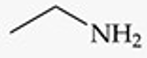
Recognize this line structure:
Amine.
What is the suffix for amines?
-amine.
What is the functional group structure of an aldehyde?
Carbonyl group (C=O) at the end of a chain, bonded to H (–CHO).
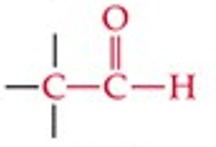
Give a simple example of an aldehyde.
Acetaldehyde (Ethanal, CH₃CHO).
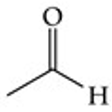
Recognize this line structure:
Aldehyde.
What is the suffix for aldehydes?
-al.
What is the functional group structure of a ketone?
Carbonyl group (C=O) bonded to two carbons.
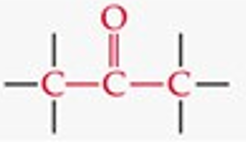
Give a simple example of a ketone.
Acetone (CH₃COCH₃).
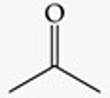
Recognize this line structure:
Ketone.
What is the suffix for ketones?
-one.
What is the functional group structure of a carboxylic acid?
–COOH (carbonyl + hydroxyl group).
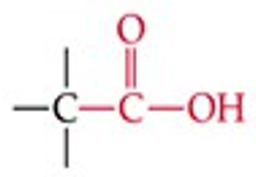
Give a simple example of a carboxylic acid.
Acetic acid (CH₃COOH).
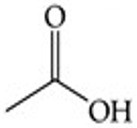
Recognize this line structure:
Carboxylic acid.
What is the suffix for carboxylic acids?
-ic acid.
What is the functional group structure of an anhydride?
Two carbonyls (C=O) bonded through an oxygen atom (–CO–O–CO–).
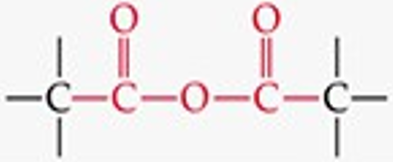
Give a simple example of an anhydride.
Acetic anhydride.
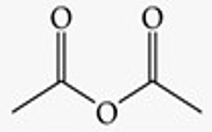
Recognize this line structure:
Anhydride.
What is the suffix for anhydrides?
None.
What is the functional group structure of an ester?
–COOR (carbonyl bonded to –OR group).
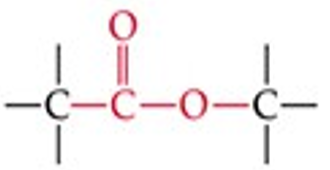
Give a simple example of an ester.
Methyl acetate (CH₃COOCH₃).
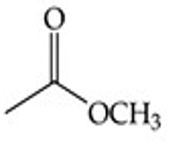
Recognize this line structure:
Ester.
What is the suffix for esters?
-ate.
What is the functional group structure of an amide?
–CONH₂ (carbonyl bonded to NH₂).
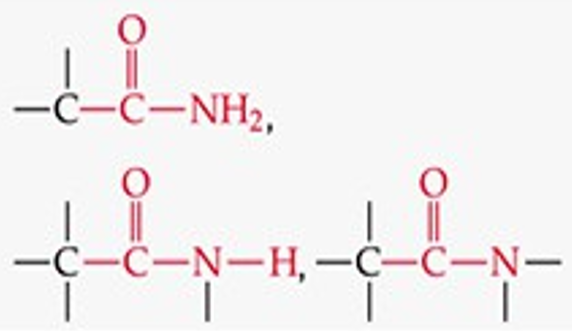
Give a simple example of an amide.
Acetamide (CH₃CONH₂).
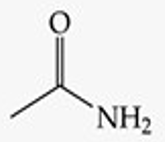
Recognize this line structure:
Amide.
What is the suffix for amides?
-amide.
What is the functional group structure of a thiol?
–SH (sulfhydryl group).
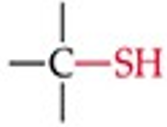
Give a simple example of a thiol.
Ethyl thiol (CH₃CH₂SH).
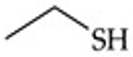
Recognize this line structure:
Thiol.
What is the suffix for thiols?
None.
What is the functional group structure of a sulfide?
R–S–R (sulfur atom bonded to two carbons).
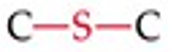
Give a simple example of a sulfide.
Ethyl methyl sulfide (CH3CH2SCH3)
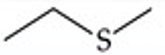
Recognize this line structure:
Sulfide.
What is the suffix for sulfides?
None.
What is the functional group structure of a disulfide?
R–S–S–R (two sulfur atoms bonded together, each bonded to carbon).

Give a simple example of a disulfide.
Dimethyl disulfide (CH₃–S–S–CH₃).
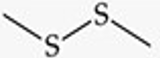
Recognize this line structure:
Disulfide.
What is the suffix for disulfides?
None.