PCOL3022 Lectures 4 & 5: GPCRs
0.0(0)
Card Sorting
1/47
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
1
New cards
The structure of GPCRs (number of transmembrane domains, residues, terminals)
- 7 transmembrane domains / anti-clockwise alpha-helices
- 25-35 hydrophobic residues
- N-terminal faces the extracellular side
- C-terminal faces the cytoplasmic side
- 25-35 hydrophobic residues
- N-terminal faces the extracellular side
- C-terminal faces the cytoplasmic side
2
New cards
How many transmembrane domains are there in GPCRs?
7
3
New cards
How many residues are there in GPCRs?
25-35 hydrophobic residues
4
New cards
Functions of GPCRs
- Sensing extracellular signals (sensory such as light, taste, odor, etc)
- Nearly all NTs interact with GPCRs (information is converted into 2nd messenger metabolite in the intracellular side)
- They are major drug targets (Involved in more than 60% of drugs)
- Activation of messenger pathways (Binding of ligands triggers cascade complex to produce biological effects and physiological responses to stimuli)
- Protein phosphorylation (Ligand binding causes conformational shifts => GRK adds phosphate groups to proteins which alters function)
- Nearly all NTs interact with GPCRs (information is converted into 2nd messenger metabolite in the intracellular side)
- They are major drug targets (Involved in more than 60% of drugs)
- Activation of messenger pathways (Binding of ligands triggers cascade complex to produce biological effects and physiological responses to stimuli)
- Protein phosphorylation (Ligand binding causes conformational shifts => GRK adds phosphate groups to proteins which alters function)
5
New cards
Identify the classes of GPCRs
Glutamate
Rhodopsin
Adhesion
Fizzled/taste 2
Secretion
Rhodopsin
Adhesion
Fizzled/taste 2
Secretion
6
New cards
Function of GluR
- Restricted to small molecules (GABA)
7
New cards
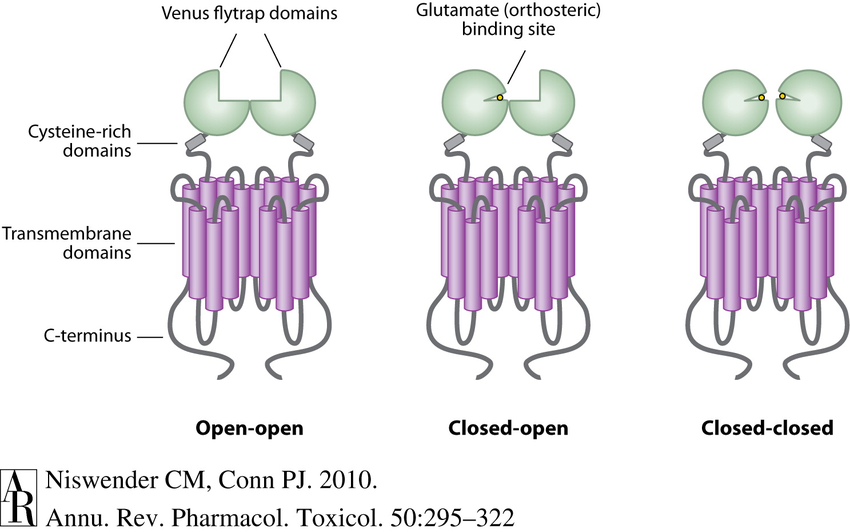
Structure of GluR Class
- Venus fly trap domain
- The N-terminus of the Glutamate receptor forms two lobes separated by a cavity (Venus fly trap domain)
- Function as dimers (covalently-linked via Cys in membrane)
- The N-terminus of the Glutamate receptor forms two lobes separated by a cavity (Venus fly trap domain)
- Function as dimers (covalently-linked via Cys in membrane)
8
New cards
Describe how the ligand activates GluR
- Glutamate binds in the cavity
- The lobes will trap the ligand like a Venus flytrap
- The lobes will trap the ligand like a Venus flytrap
9
New cards
Function of Rhodopsin Class
- Interaction with light causes cis/trans isomerism in ligand
- This activates ligand
- This GPCR protein is most abundant in rod cells (eyes)
- This activates ligand
- This GPCR protein is most abundant in rod cells (eyes)
10
New cards
Structure of Rhodopsin Class
- Ligand site is deep within the extracellular mouth
- Mouth then "closes" around ligand
- Mouth then "closes" around ligand
11
New cards
Describe how the ligand activates Rhodopsin proteins
- After the ligand binds deep inside the extracellular mouth, the pore will close
- This traps ligand in high concentration of wet volume
- This increases receptor affinity to the ligand
- This traps ligand in high concentration of wet volume
- This increases receptor affinity to the ligand
12
New cards
What are the exceptions in the Rhodopsin class?
- Growth hormones: They are large peptide receptors that have an extracellular domain that binds peptides (i.e. glycoprotein hormones)
- Protein-activated receptors (PARS): Requires hydrolysis via protease for the ligand to bind and cause activation
(Thrombin binds to N-terminal where the sequence of amino acids don't signal --> Thrombin hydrolyses in the N-terminal and creates a ligand, PAR+ for the receptor)
- Protein-activated receptors (PARS): Requires hydrolysis via protease for the ligand to bind and cause activation
(Thrombin binds to N-terminal where the sequence of amino acids don't signal --> Thrombin hydrolyses in the N-terminal and creates a ligand, PAR+ for the receptor)
13
New cards
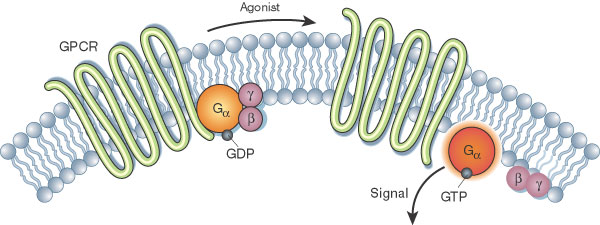
Describe the signalling mechanism of GPCRs
- The g protein is made up of three subunits -- alpha (α), beta (β) and gamma (γ)
- At resting state, these subunits are covalently bonded
- Once the ligand binds and activates the GPCR, GPD is replaced with GTP at intracellular sites
- Alpha subunit separates from the beta and gamma subunit to signal at the membrane
- Beta and gamma subunit also diffuses away to signal via the membrane
- Hydrolysis (via catalytic α-subunit): Enzyme hydrolyses GTP to GDP and all subunits for re-assembly of the complex
- At resting state, these subunits are covalently bonded
- Once the ligand binds and activates the GPCR, GPD is replaced with GTP at intracellular sites
- Alpha subunit separates from the beta and gamma subunit to signal at the membrane
- Beta and gamma subunit also diffuses away to signal via the membrane
- Hydrolysis (via catalytic α-subunit): Enzyme hydrolyses GTP to GDP and all subunits for re-assembly of the complex
14
New cards
G-Protein α Subunit Families
- Each GPCR has selectivity for an α-subunit type
- α family determines the type of signal
s (Stimulatory) - Increases cAMP formation
i/o (Inhibitory) - Decreases cAMP formation, activate or inhibit ion channels
q (Usually excitatory) - Stimulates phospholipase C then protein kinase C, and mobilises Ca2+
- α family determines the type of signal
s (Stimulatory) - Increases cAMP formation
i/o (Inhibitory) - Decreases cAMP formation, activate or inhibit ion channels
q (Usually excitatory) - Stimulates phospholipase C then protein kinase C, and mobilises Ca2+
15
New cards
Gi coupled GPCRs influence GIRK and VGIC
- Major transducers: GIRKs, VGICs
- GIRK: Beta and gamma binds to GIRK => more K+ flow out => inhibits action potential
- Ca+ VGIC: Beta and gamma interacts with Ca+ VGICs => reduce Ca2+ influx => inhibit NTs release
- G alpha i: Inhibits adenyl cyclase from producing cAMP => reduces cAMP => inhibits second messenger cascade
- GIRK: Beta and gamma binds to GIRK => more K+ flow out => inhibits action potential
- Ca+ VGIC: Beta and gamma interacts with Ca+ VGICs => reduce Ca2+ influx => inhibit NTs release
- G alpha i: Inhibits adenyl cyclase from producing cAMP => reduces cAMP => inhibits second messenger cascade
16
New cards
G-protein βγ Subunit Signalling
- Acts in membrane
- Includes modulating adenyl cyclase (cAMP formation)
- Inhibits N-type Ca2+ channels (inhibits NT release)
- Activates phospholipase C (many processes)
- Activates GIRK K+ channels (inhibits action potential activity)
- Activates PI3 kinase (many processes)
- Includes modulating adenyl cyclase (cAMP formation)
- Inhibits N-type Ca2+ channels (inhibits NT release)
- Activates phospholipase C (many processes)
- Activates GIRK K+ channels (inhibits action potential activity)
- Activates PI3 kinase (many processes)
17
New cards
Are G-α-q Coupled GPCRs excitatory or inhibitory?
Excitatory
18
New cards
G-α-q GPCR mechanism
- Gq activates phospholipase C + inositol triphosphate activation from membrane lipids
- Leads to the release of Ca2+ (stimulatory) from intracellular stores
- Leads to the release of Ca2+ (stimulatory) from intracellular stores
19
New cards
G-α-S function/mechanism
- Activates adenyl cyclase
- Increases cAMP cascade
- Increases cAMP cascade
20
New cards
G-α-i function/mechanism
- Inhibits adenyl cyclase
- Reduces cAMP cascade
- Reduces cAMP cascade
21
New cards
3 ways of GPCR signal amplification
1. Increased ligand-receptor affinity
2. Increased receptor numbers
3. Increase efficiency of transduction (GPCRs pretty efficient already)
2. Increased receptor numbers
3. Increase efficiency of transduction (GPCRs pretty efficient already)
22
New cards
What happens with increased ligand affinity?
- Improves signal detection
- Results in slow dissociation
- Therefore, high speed signalling requires low affinity ligands
- The workaround for this low affinity is signal amplification
- Results in slow dissociation
- Therefore, high speed signalling requires low affinity ligands
- The workaround for this low affinity is signal amplification
23
New cards
GPCR activity is [...] compared to LGICs
Slower
- GPCR modulation is slower compared to inotropic receptors
- Mediates fast synaptic transmission
- GPCR modulation is slower compared to inotropic receptors
- Mediates fast synaptic transmission
24
New cards
What is intrinsic efficacy?
- The ability of a receptor and drug to produce a maximal response
- Some drugs have high intrinsic efficacy than others (e.g. methadone > morphine > buprenorphine)
- Buprenorphine has a lower max response because its intrinsic efficacy is so low
- Some drugs have high intrinsic efficacy than others (e.g. methadone > morphine > buprenorphine)
- Buprenorphine has a lower max response because its intrinsic efficacy is so low
25
New cards
What will higher intrinsic efficacy produce?
- Produce a greater % receptor response
- Methadone has the greatest intrinsic efficacy compared to morphine (e.g. methadone > morphine > buprenorphine)
- Methadone has the greatest intrinsic efficacy compared to morphine (e.g. methadone > morphine > buprenorphine)
26
New cards
What does "Effective number of receptors" mean?
- How many receptors are able to be activated
27
New cards
Effects of Regulations of GPCRs
- Reduces the number of receptors
- Therefore, reduces the signalling efficacy
- Therefore, reduces the signalling efficacy
28
New cards
Process of Regulation of GPCRs
1. βγ subunits dissociate
2. GPCR is phosphorylated by GRK (a kinase)
3. Now a substrate for β-arrestin
4. β-arrestin drags the receptor to clathrin-coated pits which eventually become vesicles that ares sent to the endosome
5. From the endosome, the receptor may:
(a) eventually return to the membrane;
(b) be hydrolysed;
(c) may signal again from endosome
- This is an intrinsic feedback process
2. GPCR is phosphorylated by GRK (a kinase)
3. Now a substrate for β-arrestin
4. β-arrestin drags the receptor to clathrin-coated pits which eventually become vesicles that ares sent to the endosome
5. From the endosome, the receptor may:
(a) eventually return to the membrane;
(b) be hydrolysed;
(c) may signal again from endosome
- This is an intrinsic feedback process
29
New cards
Signalling can also occur in the [...] for GPCRs
- Some receptors can still signal from within the endosome
- Surface signal usually dominates
- Surface signal usually dominates
30
New cards
Regulation of GPCRs lead to...
- Desensitisation and tolerance in the receptor cells
- Decreased responsiveness (instrinsic feedback)
- Decreased responsiveness (instrinsic feedback)
31
New cards
Which is long term/short term: Desensitisation, tolerance
- Tolerance is long term
- Desensitisation can happen after one dose
- Desensitisation can happen after one dose
32
New cards
T/F: Once a GPCR has undergone endocytosis, it cannot be reused
False. GPCRs can be recycled
33
New cards
GPCRs structure and selectivity
- Closely related GPCRs have highly conserved structures, especially within binding pockets
- But these receptors can have vastly different physiological effects
(e.g. the adrenoreceptors)
(e.g. D2/D3 receptors:
D2/D3 antagonists are antipsychotics;
D2 mediates worst side effects;
Receptors are very similar and no selective antagonists exist yet;
Selective antagonists could probably be developed)
- But these receptors can have vastly different physiological effects
(e.g. the adrenoreceptors)
(e.g. D2/D3 receptors:
D2/D3 antagonists are antipsychotics;
D2 mediates worst side effects;
Receptors are very similar and no selective antagonists exist yet;
Selective antagonists could probably be developed)
34
New cards
A problem with GPCRs as drug targets
- Hard to discriminate pharmacologically
- Binding pockets don't always differ too much (such as D2 vs. D3 receptor)
- Binding pockets don't always differ too much (such as D2 vs. D3 receptor)
35
New cards
Selectivity from Signal Bias
- E.g. opioid receptors
- Only one receptor is involved (μOR)
- Different agonists have different combinations of G-protein and arrestin signalling
- Structural bias not yet understood
- Established in many GPCRs
- Only one receptor is involved (μOR)
- Different agonists have different combinations of G-protein and arrestin signalling
- Structural bias not yet understood
- Established in many GPCRs
36
New cards
Differences in downstream signalling lead to [...] bias
Signal bias
37
New cards
What does 'biased signalling' mean?
- Different agonists induce differing conformations and initiate different signalling cascades
38
New cards
Comparison example of biased signalling
- Endomorphin-2 produces moderate G-protein signal and strong arrestin signal
- Methadone produces strong G-protein signal and strong arrestin signal
- Methadone produces strong G-protein signal and strong arrestin signal
39
New cards
Orthosteric agonism
- Agonist binds in endogenous ligand's binding pocket
40
New cards
Allosteric agonism
- Ligand binds in alternative area ("allo" means "other")
- Can be positive, neutral, or negative
- Can be positive, neutral, or negative
41
New cards
Why is selectivity high for allosteric modulators of GPCRs?
- The binding site is remote from the orthostatic site
- There is more divergence in allosteric binding sites, meaning the greater potential for selective PAMs that can be used therapeutically
- E.g. Ca-sensing receptor PAM (positive allosteric modulators) -- increases agonist affinity and/or efficacy -- helps increase the sensitivity of receptor -- treats hyperthyroidism
- There is more divergence in allosteric binding sites, meaning the greater potential for selective PAMs that can be used therapeutically
- E.g. Ca-sensing receptor PAM (positive allosteric modulators) -- increases agonist affinity and/or efficacy -- helps increase the sensitivity of receptor -- treats hyperthyroidism
42
New cards
[...] will produce signalling which can be increased by [...]
- Orthosteric agonism will produce signalling which can be increased by positive allosteric modulator (PAM)
- A negative allosteric modulator (NAM) will decreased GPCR signalling
- A negative allosteric modulator (NAM) will decreased GPCR signalling
43
New cards
Are greatest structural constraints for drug manufacture at the orthosteric or allosteric site?
Orthosteric
44
New cards
Selectivity from Dimerisation
- Most GPCRs exist as dimers
- Can possibly develop selective drugs from certain heterodimer combinations
- Unclear if these heterodimers do exist in vivo
- E.g. GABAB receptor requires both GABA1 and GABA2.
GABA1 is responsible for binding GABA,
While GABA2 is responsible for transduction of signal
- Can possibly develop selective drugs from certain heterodimer combinations
- Unclear if these heterodimers do exist in vivo
- E.g. GABAB receptor requires both GABA1 and GABA2.
GABA1 is responsible for binding GABA,
While GABA2 is responsible for transduction of signal
45
New cards
DREADDs
Designer Receptor Exclusively Activated by Designer Drugs
46
New cards
What are DREADDs?
- Proteins manipulated to react specifically with small molecules which act as chemical actuators, but which were not previously recognized by these proteins
47
New cards
Example of DREADDs
- Created mutant MR (mutated at TM3, TM5)
- Doesn't respond to MR agonists
- Responds to novel agonist CNO, which doesn't act on any other GPCRs
- Doesn't respond to MR agonists
- Responds to novel agonist CNO, which doesn't act on any other GPCRs
48
New cards
Optogenetics example of DREADDs
Channelrhodopsin (ChR2) activates in response to light