UWorld-General Chemistry-Thermochemistry, Kinetics & Gas Laws (Kapllan Ch's 5, 7-8)
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
What's the relationship between the slope and ΔH when given a Van't hoff plot?
It's an inverse relationship!
If the slope is negative, then ΔH is POSITIVE.
If the slope is POSITIVE, then ΔH is NEGATIVE.
This can be seen mathematically, see notes for formula.
What's the equation that relates Gibb's free energy to the equilibrium constant (Keq)?

What happens to ΔG as we INCREASE T and Keq?
ΔG = -RTln Keq
It DECREASES. This is because if we multiply -R which is a negative value, by a larger number for T and K eq, the G will increase.
What happens to ΔG as we DECREASE T and Keq?
ΔG = -RTln Keq
It INCREASES
Entropy (formula, positive entropy and negative entropy meanings)?
ΔS universe = ΔS System + ΔS Surroundings
+ ΔS = Spontaneous reaction (*universe favors an increase in entropy).
- ΔS = Non-spontaneous reaction
What kind of property is entropy? What does this mean?
Entropy is an extensive property. This means that it increases as the amount of a substance (volume, size, temperature, number of molecules) increases.
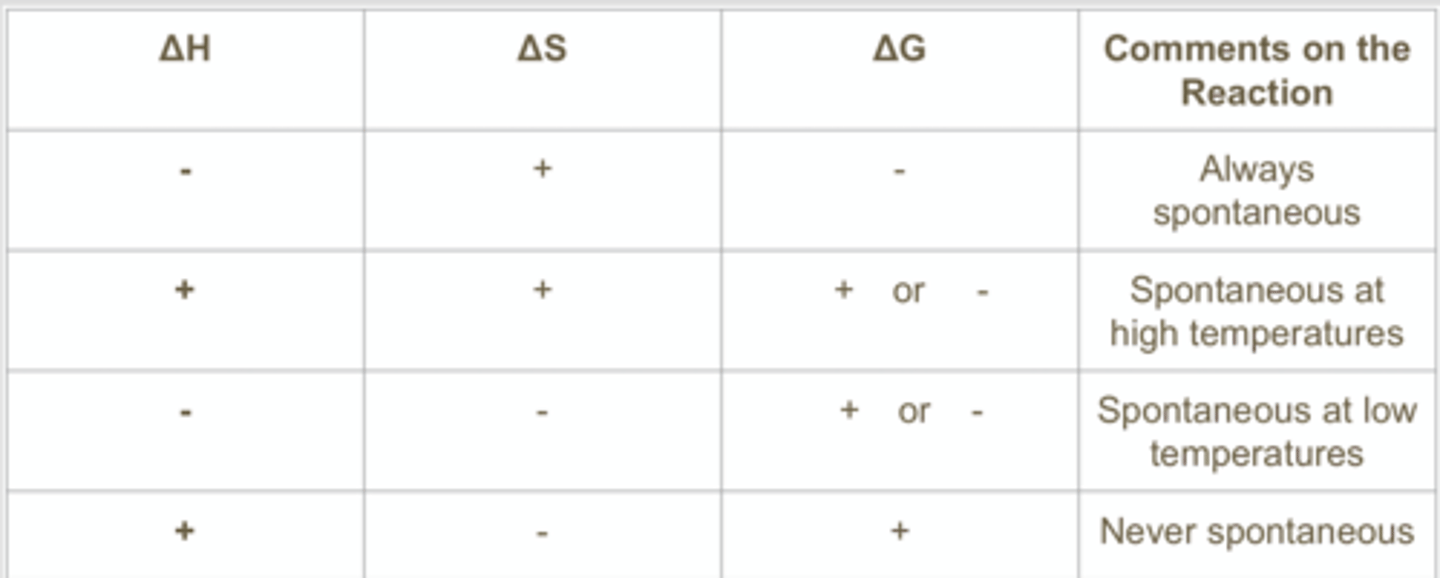
Gibbs free energy chart
Ka =
Acid dissociation constant (equilibrium constant, this excludes liquids and solids).
Ex:
HA + H2O ↔ H+ + A-
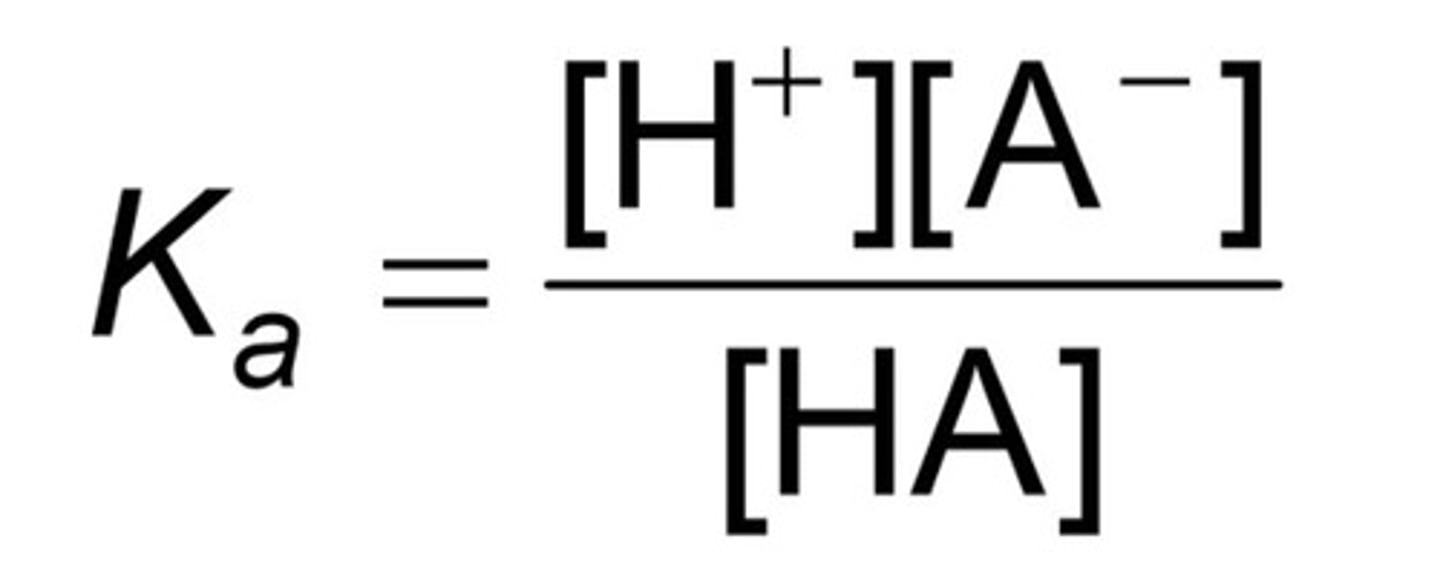
pH = ?
−log[H+]
pOH = ?
−log[OH+]
pH + pOH = ? = ?
14, pKw
Do enzymes affect the overall ΔG or ΔH of a reaction?
No, because these are thermodynamic values (state functions) that are independent of the chemical pathway that a reaction takes to get from reactants to products.
They can only tell us about the current state of products they can't tell us how they got there.
What factors affect the reaction rate of a reaction?
1. Reaction concentrations
2. Temperature
3. The medium in which the reaction takes place and the physical state of the medium (Polar solvents are preferred).
4. Catalysts
How does the reaction concentrations affect the reaction rate?
The greater the concentration of the reactants, the more collisions per unit time
What are all the state functions?
When i'm under pressure and feeling dense all I want to do is watch TV and get HUGS.
Pressure (P), density (p), Temperature (T), volume (V), enthalpy (H), internal energy (U), gibbs free energy (G), and entropy (S).
Do enzymes alter the Keq?
No (i.e. Ka, Kb values).
Enzymes do not impact the __________ of a biological reaction?
Thermodynamics (ΔG, ΔS, ΔH).
Is a dissociation reaction endothermic or exothermic? Why?
It's endothermic because in order for us to "dissociate" or break apart a bond, we must have energy (heat) to do so.
How do dissociation reactions (i.e. dissociation of an acid/base) affect the entropy of a reaction?
They INCREASE THE ENTROPY because they break large molecules into smaller ones (going from fewer molecules to more), which increases the disorder.
What can be said about the energy of the reactants and products in an ENDOTHERMIC reaction?
The energy of the products is HIGHER than the energy of the reactants since we ABSORBED energy.
What can be said about the energy of the reactants and products in an EXOTHERMIC reaction?
The energy of the reactants is HIGHER than the energy of the products since we RELEASED energy.
In order to break apart bonds/molecules we need to ______energy into them?
Put energy into them; endothermic reaction.
Think of someone trying to break apart a couple who has been married for a long time, we would have to put a lot of energy into breaking them apart.
In order to FORM BONDS, we need to ________ energy?
Release energy; exothermic reaction.
Think of a matchmaker that wants to bring a couple together and once they're finally together, they express their love (energy) to the world by shouting it out (releasing it).
Rate law formula
ONLY INCLUDES THE REACTANTS.
A and B are the reactants in the reaction and x and y are the orders of the reaction.
aA + bB -------> cC + dD
= rate = k [A]^x[B]^y
![<p>ONLY INCLUDES THE REACTANTS.</p><p>A and B are the reactants in the reaction and x and y are the orders of the reaction.</p><p>aA + bB -------> cC + dD</p><p>= rate = k [A]^x[B]^y</p>](https://knowt-user-attachments.s3.amazonaws.com/c702b30d-622a-4db8-8217-4bec54dbe97f.jpg)