Chem hybridization
1/49
Earn XP
Description and Tags
CHEM1270
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Alkanes
single bonds
Alkenes
double bonds
Alkynes
triple bonds
single bond is what hybridization?
sp3
double bond is what hybridization?
sp2
triple bond is what hybridization?
sp
Meth-
one carbon atom
Eth-
two carbon atoms
Prop-
three carbon atoms
But-
four carbon atoms
Pent-
five carbon atoms
Hex-
six carbon atoms
Hept-
seven carbon atoms
Oct-
eight carbon atoms
Non-
nine carbon atoms
Dec-
ten carbon atoms
Isomers
compounds with same molecular formula but different structural formulas
Sublimation
solid to gas (endothermic)
Deposition
gas to solid (exothermic)
Fusion
melting, solid to liquid (endothermic)
Freezing
liquid to solid
dipole-dipole
polar molecules attract one another intermolecular interaction
London dispersion
Attraction between temporary induced dipoles in neighboring molecules
Hydrogen bonding
Intermolecular interaction when H is bonded to very electronegative elements, N, O, and F
critical pressure
the pressure at which transition occurs
Vapor pressure
energy of a system
Le Chatelier’s principle
when dynamic equilibrium is disturbed, the system responds to minimize the disturbance and returns to the state of equilibrium
Boiling point
the point when the liquid’s vapor pressure equals the external pressure
normal boiling point’s vapor pressure
1 atm
Dynamic equilibrium
the rate of condensation and the rate of vaporization becomes equal
Crystalline
atoms or molecules arranged in a well-ordered three-dimensional array
miscibility
another word for solubility
Intermolecular forces
the forces that hold condensed states together
amorphous
atoms or molecules have no long-range order
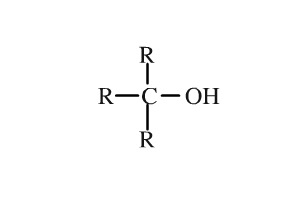
Alcohol
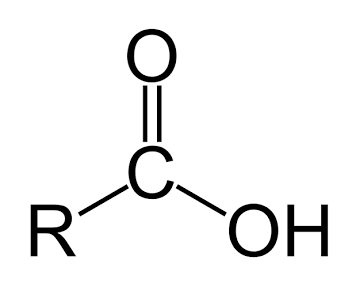
Carboxylic Acid
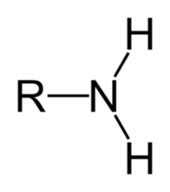
Amine
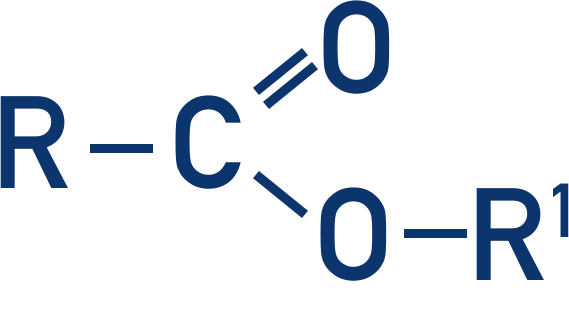
Ester
Amide
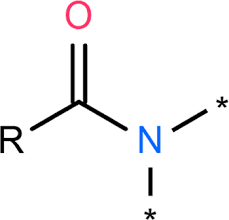
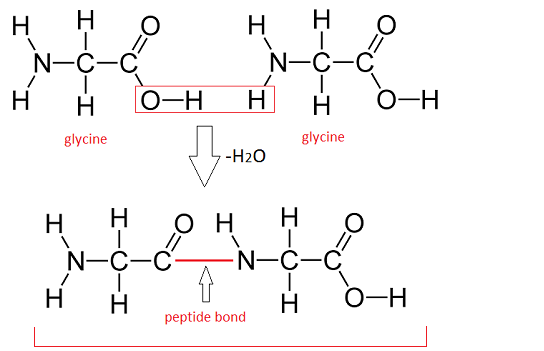
Dipeptide reaction
Ester
smells nice, found in nature
CH2-CH3
ethyl group
CH3
methyl group
enantiomers/chiral
molecules that are different than their mirror images, have 4 different atoms/groups attached to a carbon atom with tetrahedral molecular geometry
sp
linear
sp2
trigonal planar
sp3
tetrahedral
sigma bond form in what number bonds?
single bonds
pie bonds form in what number bonds?
double and triple bonds