kinetic model of matter
0.0(0)
Card Sorting
1/5
Last updated 7:55 AM on 10/13/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
brownian motion
states that all matter is made up of a large number of small particles which are in continuous and random motion
2
New cards
brownian motion: types of particles and why they move
suspended particles: eg smoke particles
fluid particles: eg air molecules
fluid particles undergo continuous and random motion, collide with suspended particles continuously
fluid particles: eg air molecules
fluid particles undergo continuous and random motion, collide with suspended particles continuously
3
New cards
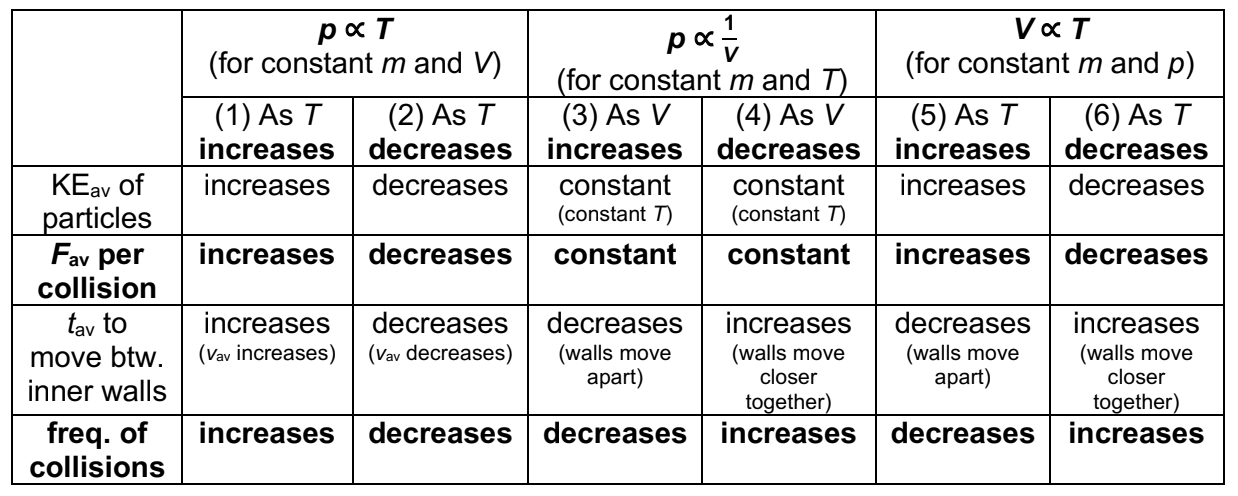
gas laws:
pressure - temperature
pressure - temperature
as temperature increases, average KE of gas particles increases
average force per collision by gas particles on the inner walls of the container increases
frequency of collisions increases
thus, gas pressure increases
average force per collision by gas particles on the inner walls of the container increases
frequency of collisions increases
thus, gas pressure increases
4
New cards
pressure - volume
since temp is constant, KE is constant
average force per collision is constant
as volume of container increases, number of gas particles per unit volume decreases
frequency of collisions decreases
gas pressure decreases
average force per collision is constant
as volume of container increases, number of gas particles per unit volume decreases
frequency of collisions decreases
gas pressure decreases
5
New cards
volume - temperature
temp increases, average KE increases
average force per collision by gas particles on inner walls increase
as volume increases, number of gas particles per unit volume decreases
frequency of collisions decreases
these two counteracting factors ensure pressure is constant
average force per collision by gas particles on inner walls increase
as volume increases, number of gas particles per unit volume decreases
frequency of collisions decreases
these two counteracting factors ensure pressure is constant
6
New cards
summary