Electrolytes in Clinical Chemistry: Food Sources and Functions
1/240
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
241 Terms
Osmolality
Physical property of a solution based on the concentration of solutes, expressed as millimoles per kilogram of solvent (w/w).
Electrolytes
Ions capable of carrying an electric charge, found in blood, urine, tissues, and other body fluids.
Functions of Electrolytes
Regulate volume and osmotic balance, myocardial rhythm, enzyme activation, and acid-base balance.
Normal plasma osmolality
275 - 295 mOsm/kg of plasma H2O.
Sodium Food Sources
Processed foods, cheese, breads, cereals, sauces, pickled foods, commercial rice or pasta mixes, and condiments.
Potassium Food Sources
Green leafy vegetables (spinach, kale), tomatoes, cucumbers, pumpkin, carrots, potatoes, banana, avocado, beans, peas, milk, and yoghurt.
Chloride Food Sources
Seafood, seaweeds, rye, tomatoes, lettuce, celery, and olives.
Calcium Food Sources
Milk, milk alternatives, soya, nuts, and green leafy vegetables (broccoli, cabbage, okra).
Magnesium Food Sources
Legumes, nuts, seeds, fish, and whole grains.
Functions of Na, Cl, K
Volume and osmotic regulation.
Functions of K, Ca, Mg
Myocardial rhythm and contractility.
Functions of Ca, Mg, Zn, K, Cl
Important cofactors in enzyme activation.
Functions of Mg
Regulation of ATPase ion pumps.
Functions of K, Ca, Mg
Neuromuscular excitability.
Functions of Mg, PO₄
Production and use of ATP from glucose.
Functions of HCO3, K, Cl, PO4
Maintenance of acid-base balance.
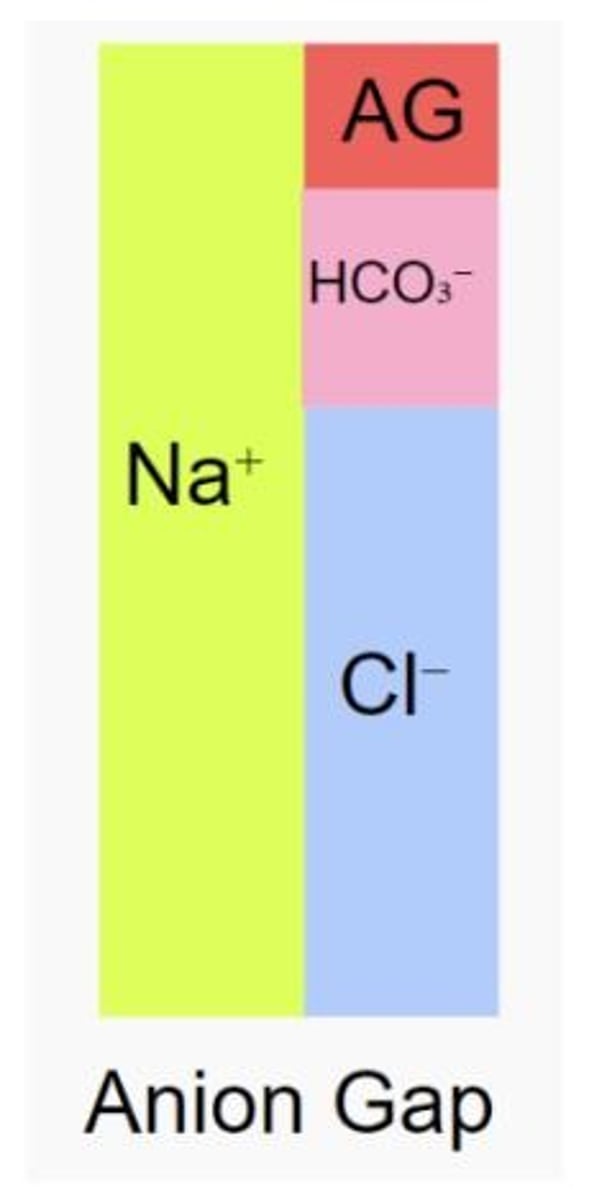
Anion Gap Formula
Anion Gap = Na+ - (Cl- + HCO3-).
Osmolality Formula
Osmolality = Total moles of solute (millimoles) / Weight of solvent (kg).
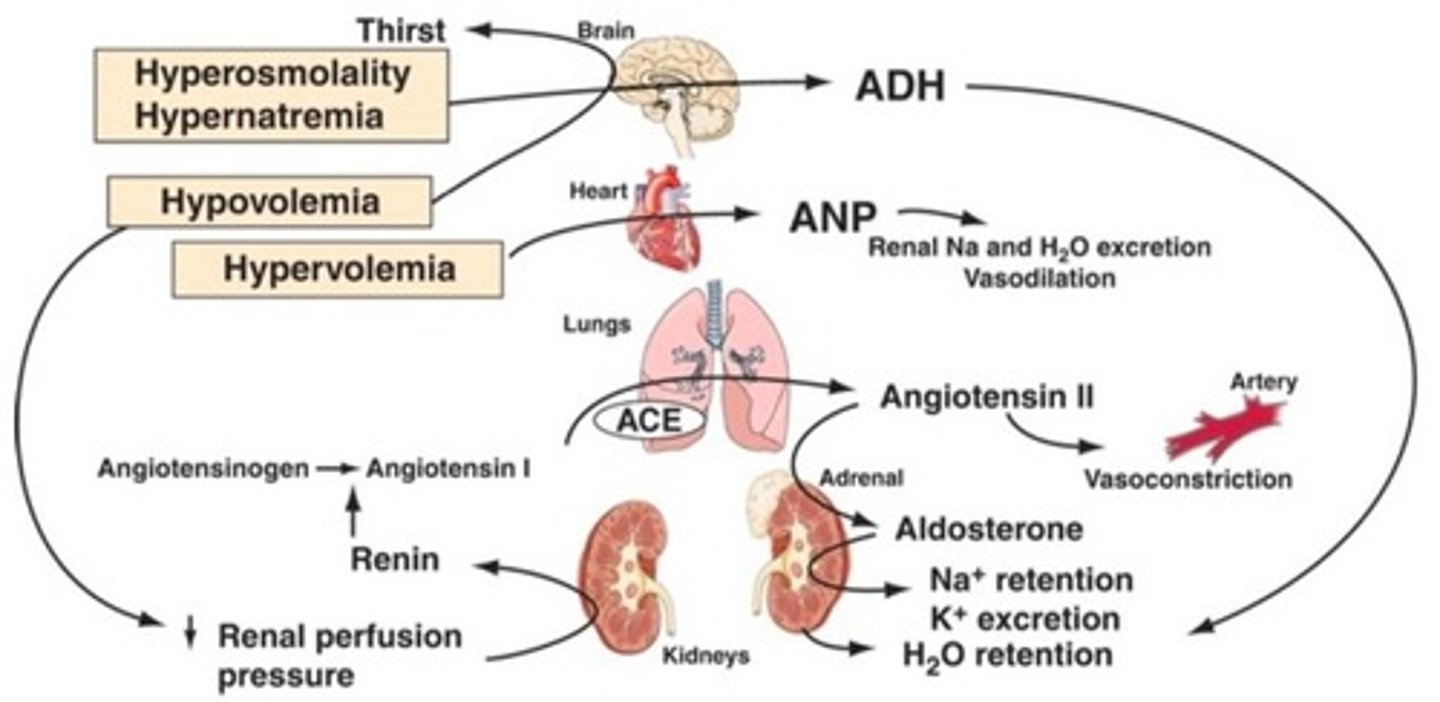
AVP Response to Osmolality
A 1-2% increase in osmolality causes a fourfold increase in the circulating concentration of Arginine Vasopressin (AVP).
Electroneutrality
Fluids always contain equal numbers of cations and anions.
Disassociation of Solutes
Depends on the chemical composition of the compound and the concentration of other charged particles in the medium.
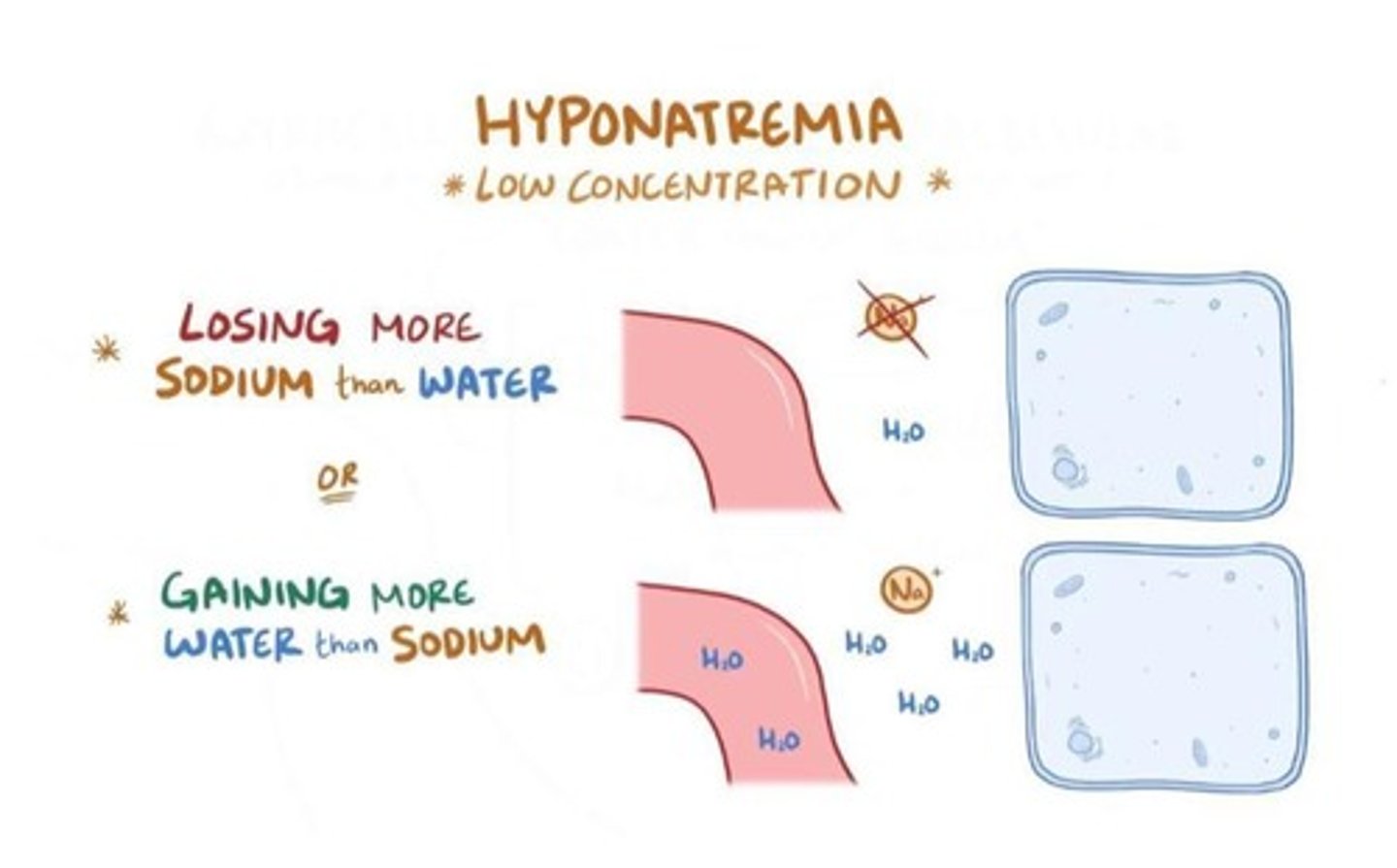
Hyponatremia
A condition characterized by low sodium levels in the blood.
Pseudohyponatremia
A laboratory artifact that results in falsely low sodium levels due to high lipid or protein levels.
Hypernatremia
A condition characterized by high sodium levels in the blood.
Serum Osmolality
275-295 mOsm/kg
Urine Osmolality (24h)
300-900 mOsm/kg
Urine/Serum Ratio
1.0-3.0
Random Urine Osmolality
50-1,200 mOsm/kg
Osmolal Gap
5-10 mOsm/kg
Hyponatremia
Low concentration of sodium <135 mmol/L, clinically significant if <130 mmol/L
Abnormal Blood Sodium Levels
Changes in blood sodium levels affect body water content, causing dehydration or edema.
Natrium
Present in all body fluids, found in highest concentration in blood and extracellular fluid.
Major extracellular cation
Sodium is a major contributor to osmolality and helps maintain osmotic pressure.
Sodium Regulation Processes
Intake of water in response to thirst, excretion of water, blood volume status.
Increased Na+ Loss Causes
Hypoadrenalism, potassium deficiency, diuretic use, ketonuria, salt-losing nephropathy, prolonged vomiting or diarrhea, severe burns.
Increased Water Retention Causes
Renal failure, nephrotic syndrome, hepatic cirrhosis, congestive heart failure, excess water intake.
Water Imbalance
Can be due to vasopressin hormone secretion or syndrome of inappropriate arginine vasopressin (SIADH).
Classification of Hyponatremia
Can be classified according to serum/plasma osmolality: low, normal, or high osmolality.
Low Osmolality Hyponatremia Causes
Increased sodium loss, increased water retention, increased non-sodium cations, lithium excess, increased gamma globulins-cationic.
Normal Osmolality Hyponatremia Causes
Severe hyperkalemia, severe hypermagnesemia, severe hypercalcemia, pseudohyponatremia, hyperlipidemia, hyperproteinemia.
High Osmolality Hyponatremia Causes
Hyperglycemia, mannitol infusion.
Pseudohyponatremia
Reduction of serum sodium concentration due to systematic error in measurement, often caused by excess lipids in serum.
Symptoms of Hyponatremia
125-130 mmol/L: GI symptoms; <125 mmol/L: nausea, vomiting, muscular weakness, headache, lethargy, ataxia; severe cases can lead to seizures, coma, and death.
Acute Hyponatremia
Considered a medical emergency if serum sodium <120 mmol/L for 48h or less.
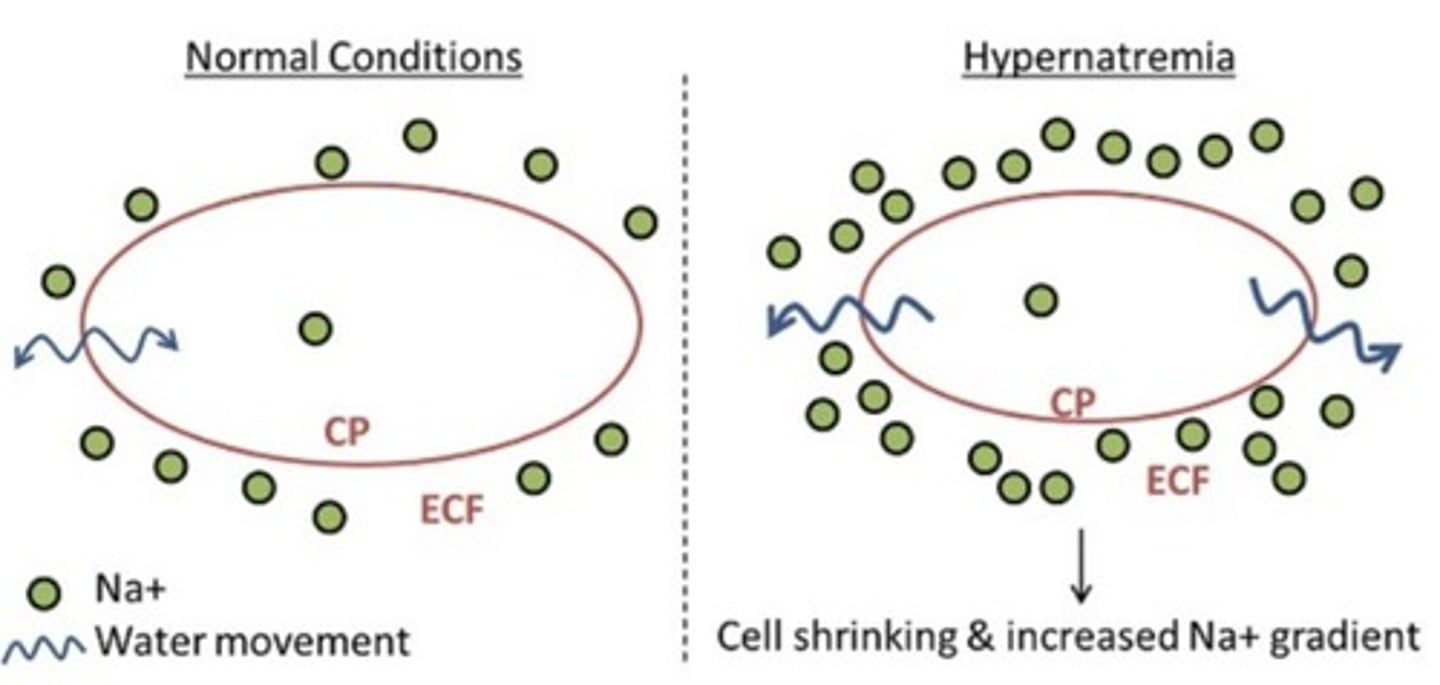
Hypernatremia
High sodium levels in the blood.
Sodium level
>145 mmol/L.
Mortality rate at sodium level >160 mmol/L
Associated with a mortality rate of 60-75%.
Causes of Hypernatremia
Due to excessive loss of water relative to Na+ loss, decreased water intake, or increased Na+ intake or retention.
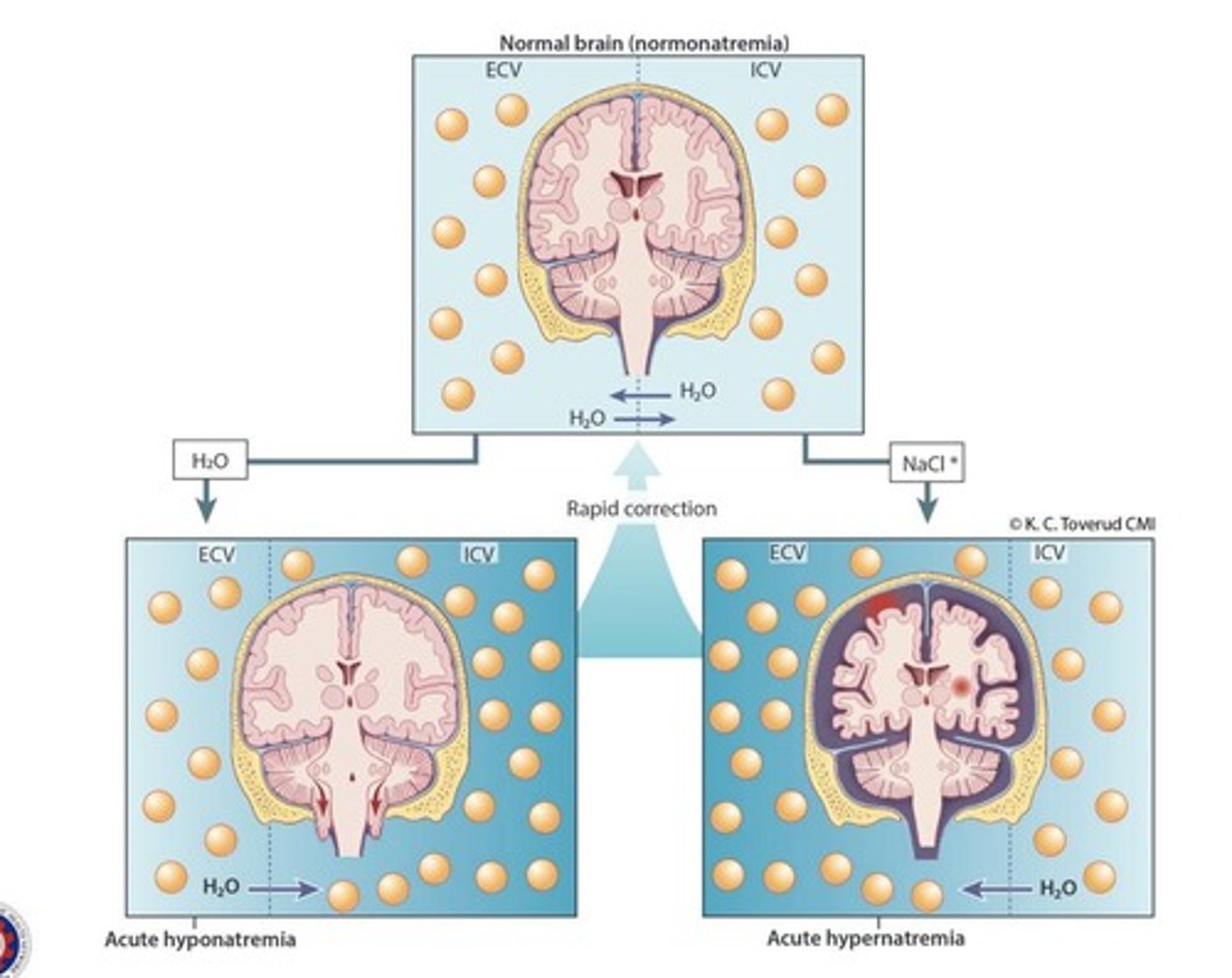
Correction of sodium levels
Should be corrected very slowly since the brain is very sensitive to water movement.
Hypoosmolality
Low sodium levels in the blood and low sodium levels in the brain.
Common Treatment for Hypoosmolality
Give sodium (~10 mmol per 24 hours).
Osmolar Demyelination Syndrome (ODS)
Occurs when sodium is rapidly supplied to the blood vessels, causing water to travel from the brain to the blood vessels.
Hyperosmolality
High sodium levels in the blood and high sodium levels in the brain.
Common Treatment for Hyperosmolality
Give water.
Cerebral edema
Occurs when water is rapidly supplied to the blood vessels, causing water to travel from the blood vessels to the brain.
Fast correction of sodium levels
Can cause brain injury.
Chronic hypernatremia in alert patients
Indicative of a hypothalamic disease.
Adipsia
Inadequate water intake due to hypothalamic dysfunction.
Sodium determination specimen types
Serum, plasma, urine (24h), sweat.
Ion-Selective Electrode (ISE)
Most routinely used method for sodium determination.
Diabetes insipidus
Characterized by copious production of dilute urine (3 to 20L/day).
Reference range for serum/plasma sodium
135-145 mmol/L.
Reference range for urine sodium (24h)
40-220 mmol/d, varies with diet.
Causes of dehydration leading to hypernatremia
Not drinking enough fluids, diarrhea, kidney dysfunction, diuretics, excessive sweating, hormonal imbalance.
Cerebrospinal Fluid
135-150 mmol/L
Hypernatremia Classification by Urine Osmolality
< 300 mOsm/kg
Hypernatremia Classification by Urine Osmolality
300-700 mOsm/kg
Hypernatremia Classification by Urine Osmolality
> 700 mOsm/kg
Potassium
Major intracellular cation
Potassium
Mainly from fruits and vegetables
Potassium
Has a blood pressure-lowering effect
Potassium Functions
Regulation of neuromuscular excitability
Potassium Functions
Contraction of the heart
Potassium Functions
ICF volume
Potassium Functions
H+ concentration
Plasma K+ Effect
Affects the resting membrane potential (RMP) of the cell (RM is closer to zero), thereby affecting cell excitability
Potassium Sources
Obtained from food and drink and loses it primarily in urine, some in the digestive tract and sweat
Potassium and Muscle Contraction
For muscle contraction, particularly of the heart muscles
Potassium Chloride Use
Typically used in death penalty cases to induce heart arrhythmia
Potassium Regulation
Dietary deficiency or excess is rarely a primary cause of hypo-/hyperkalemia; instead, it enhances the degree of the disorder → cause is mostly due to a preexisting condition
Symptoms of Potassium Imbalance
Usually involve the CNS
Symptoms of Potassium Imbalance
Altered mental status
Symptoms of Potassium Imbalance
Lethargy
Symptoms of Potassium Imbalance
Irritability
Symptoms of Potassium Imbalance
Restlessness
Symptoms of Potassium Imbalance
Seizures
Symptoms of Potassium Imbalance
Muscle twitching
Symptoms of Potassium Imbalance
Hyperreflexia
Symptoms of Potassium Imbalance
Fever
Symptoms of Potassium Imbalance
Nausea or vomiting
Symptoms of Potassium Imbalance
Difficult respirations
Symptoms of Potassium Imbalance
Increased thirst
Aldosterone
Hormone produced by the adrenal glands in the kidneys that mainly controls levels.
K+ Loss
Occurs whenever the Na+, K+-ATPase pump is inhibited by conditions such as hypoxia, hypomagnesemia, or digoxin overdose.
Digoxin
A drug used in the pediatric population with heart failure or reduced pumping ability to stimulate the heart.
Insulin
Promotes acute entry of K+ into skeletal muscle and liver by increasing Na+, K+-ATPase activity; used in hyperkalemia.
Catecholamines
Such as epinephrine (β2-stimulator), promote cellular entry of K+.
Propranolol
A β-blocker that impairs cellular entry of K+.
Exercise
Causes K+ release from muscle cells, reversible after several minutes of rest.
Hypernatremia
Must be corrected gradually; too rapid correction can induce cerebral edema and death.