Unit 3 Part 2 - Properties of Substances & Mixtures
1/42
Earn XP
Description and Tags
Covers the second half of Unit 3 of AP Chemistry (3.7-3.13)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
43 Terms
What is a Homogeneous Mixture?
A physical combination of any state of matter where macroscopic (big) properties (density, texture, color, etc) do not vary.
Example: Chicken broth, salt water. (can be liquid, solid, gas)
What is a heterogeneous mixture? What does this mean about its macroscopic properties?
A mixture where there the composition is not uniform throughout.
This means that its properties, like density, color, texture, can be different depending on how you look at it.
What is the idea of Solvation?
It is when the solute and solvent orient themselves based on their IMFs when they are mixed together.
How do you find the volume of a solvent or solute?
The volume of the solvent would be the entire solution since it’s not considered.
What happens when Ionic Solutes are dissolved?
It dissociates (separates) into its component ions.
What happens when a molecular solute dissolves?
Its molecules separate (because you break up the IMFs) and evenly disperse throughout the solution.
What happens to the volume of a solution when you add a solute?
It is typically a small change because the solvent will occupy the space between the solute particles (recall the idea of solvation).
What are particulate models?
They display both the relative concentrations and interactions among components.
What is the use of particulate models?
They are used to represent the interactions between components of a mixture like ion sizes, or the orientation of solute ions and solvent particles.
It’s the things we had to draw in the lab where we had to draw water molecules with NaCl2!
What does the dilution formula tell us (M1V1= M2V2)?
It can calculate the volume/molarity of a newly diluted solution or can calculate what volume of the original solution is needed to a volume of a new solution at a new molarity.
What needs to happen when you dissolve a solute in a solvent?
The solute-solute IMFS must be broken which takes energy.
What needs to happen to the solvent when you want to dissolve a solute?
The solvent-solvent IMS must be broken which takes energy.
What happens, after breaking both the solute and solvent’s, when you form a solution?
Newly formed IMFS will be created between the solvent and solute, releasing energy.
What is “like dissolves like?”
The idea that it is more energy favorable for a molecule to separate and reform with new molecules (why solutions are a thing).
Why will solutions sometimes not form?
Because the energy required to separate both the solute and solvent so much greater than the energy released by solvation.
Why won’t solutions separate through filtration?
It is beause their IMFS have to be broken.
How does distillation work? (a way to separate solutions)
It uses varying strength of IMFs between the components and will separate theem based on the vapor pressures of the components in the mixture.
Example: When water starts to boil, the lighter stuff will boil first and will go towards the top of a container
When should you pick chromatography over distillation or vice versa?
Chromatography is better for separating things with similar boiling points.
Distillation is better for things that easily turn into gasses.
Describe the process of chromatography…
It relies on the IMFS between the solution and stationary things (like a piece of paper/things packed into a column). Basically, the IMFs interact with that stationary phase and get stuck in that phase while the rest of the solution keeps moving.
What is column chromatography?
It results in separate liquid samples of each component.
What is paper chromatography?
It leaves the separated components on the paper.
What is the mobile phase in paper chromatography?
It is the solvent that carries the molecule being tested up the stationary part.
What is the solvent front?
The furthest distance traveled by the solvent (the highest line on a graph).
What is the retention factor?
It determines how far something went up a paper chromatography graph.
How do you calculate the retention factor?
It’s the distance traveled by the compound divided by the distance traveled by the solvent front (both measured from the origin).
In paper chromatography, what happens as things move up the paper?
Molecules with stronger IMFs will stop traveling more quickly than molecules with weaker IMFs (they will be at the top).
What three things affect how fast molecules travel up in chromatography?
Polarity (more attracted to itself than other stuff), molecular size, ion charges.
How are aqueous and liquid solutions best separated?
By evaporating the water from the solution.
What affects how soluble something will be?
Substances with similar IMFs will tend to be soluble with one another.
In what kind of substance will ionic molecules dissolve in? Why????
They will dissolve in polar solvents because cations interact with the negative’s in water molecules and the anions interact with positive water molecules.
When will molecular substances dissolve? Why????
What happens with larger molecules?
They will dissolve in nonpolar solvents because they have LDFs.
The larger and more polarizable the electron cloud, the more interactions that will occur with the solvent.
What is spectroscopy?
The idea that you can find out what elements are in something based on the signature of ultraviolet/visible light they give out.
How do elements give off light?
The motion of the atoms AND their electrons results in the absorption/emission of photons (with quantized energy).
EX: If an electron jumps from an energy level, it needs an exact amount of energy and releases an energy (which is light).
How does an ATOM give off light?
When electrons transition energy levels and ultraviolet/visible radiation is absorbed/emitted.
How do molecules give off light? Hint: there’s two ways
As the atoms rotate along a bond, they transition between energy levels and microwave radiation is absorbed/emitted.
As the atoms vibrate along the bond, they transition between energy levels and infrared radiation is absorbed/emitted.
In terms of light waves, which one is the shortest? Longest? give your answer with the nm too
Longest would be red (750nm)
Shortest would be purple (400nm)
What is the equation that relates wavelength, frequency, and speed of light?
Speed of Light = Wavelength x frequency
What equation can you use when energy behaves as a particle (a photon)?
Ephoton = Planck’s Constant * Frequency
What happens if a photon at a specific frequency hits the surface of a structure (particularly a metal)?
It will knock electrons off the surface IF the frequency is enough for the electron to move across quantized energy levels.
What is the beer-lambert law equation?
A=εbc
A = Absorbance of light
ε = molar absorptivity; the degree to which a solution absorbs light
c = concentration
b = path length
What happens if path length and wavelength are held constant?
Then absorbance and concentration are proportional.
List the complementary colors for the spectrophometer:
Yellow and Purple
Red and Green
Blue and Orange
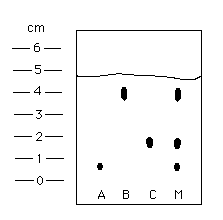
Calculate the Retention Factors for the image listed!
For A: 0.7/4.7 = 0.1489
For B: 4/4.7 = 0.8511
For C: 1.8/4.7 = 0.3830
For M: Not needed :)