4c: Alkanes
5.0(1)
5.0(1)
Card Sorting
1/23
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
1
New cards
alkane
saturated hydrocarbons
2
New cards
saturated - definition
only single carbon-carbon bonds
3
New cards
general formula for alkanes
**C**n**H**2n+2
4
New cards
properties of alkanes
* colourless compounds
* gradual change in physical properties as carbon atoms increase
* generally unreactive
* undergo combustion
* can be cracked into smaller molecules
* can react with halogens in the presence of light
* gradual change in physical properties as carbon atoms increase
* generally unreactive
* undergo combustion
* can be cracked into smaller molecules
* can react with halogens in the presence of light
5
New cards
first 5 members of the alkane homologous series
1. methane
2. ethane
3. propane
4. butane
5. pentane
6
New cards
uses - methane, ethane, propane
* methane: major component of natural gas
* ethane, propane: present in natural gas and LPG
* ethane, propane: present in natural gas and LPG
7
New cards
LPG
liquefied petroleum gas
8
New cards
methane - molecular formula & structural formula
**CH**4
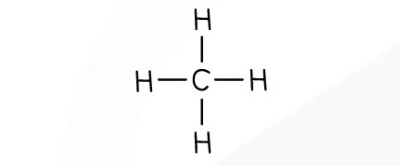
9
New cards
ethane - molecular formula & structural formula
**C**2**H**6
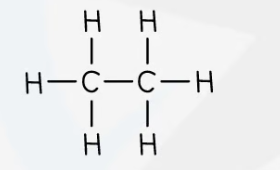
10
New cards
propane - molecular formula & structural formula
**C**3**H**8
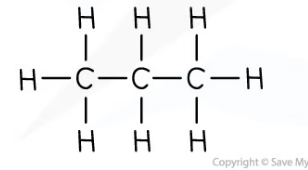
11
New cards
butane - molecular formula & structural formula
**C**4**H**10
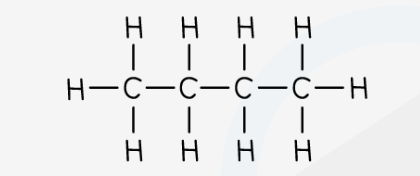
12
New cards
pentane - molecular formula & structural formula
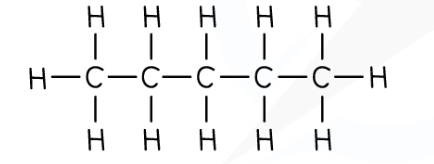
**C**5**H**12
13
New cards
what happens in a substition reaction
a halogen atom takes place of a hydrogen atom
14
New cards
what has to be present for alkanes to undergo a substitution reaction with halogens
UV radiation
15
New cards
methane and bromine - substitution reaction in the presence of UV radiation (symbol equation)
**CH**4 + **Br**2 → **CH**3**Br** + **HBr**
16
New cards
methane and bromine - substitution reaction in the presence of UV radiation (word equation)
methane + bromine → bromomethane + hydrogen bromide
17
New cards
methane and chlorine - substitution reaction in the presence of UV radiation (symbol equation)
**CH**4 + **Cl**2 → **CH**3**Cl** + **HCl**
18
New cards
methane and chlorine - substitution reaction in the presence of UV radiation (word equation)
**methane + chlorine → chloromethane + hydrogen chloride**
19
New cards
which family do the products of the substitution reactions in the presence of UV radiation belong to
halogenoalkanes / haloalkanes
20
New cards
CFC
chlorofluorocarbon - a class of halogenoalkanes
21
New cards
isomers
compounds with the same molecular formula but different structural formulae
22
New cards
properties of a homologous series
* same functional group
* similar chemical properties
* trend in physical properties
* described by same general formula
* differ from the next by **CH**2
* similar chemical properties
* trend in physical properties
* described by same general formula
* differ from the next by **CH**2
23
New cards
free radical
the atoms formed when substitution reactions occur due to the presence of unpaired electrons
24
New cards
mono-substitution
when only one hydrogen atom in the alkane is replaced by a halogen atom