RRoy 3: Regulatory Sequences in RNA Polymerase II Promoters
1/36
Earn XP
Description and Tags
3rd rick roy lecture
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
Why is the CTD domain important in yeast?
If you eliminate it in yeast, they cannot grow
RNA polymerase II needs this domain to carry out it’s function
Heptapeptide repeat definition and example
Sequence of 7 amino acids arranged in a specific sequence that get repeated
Ex. Carboxyl Terminal Domain - This leads to the unstrctured appearance of CTD domains
How many CTD heptapeptide repeats are found in humans? How many in yeast?
In humans there are 52 heptapeptide repeats
In yeast, there are 26 (yeast need a minimum of 10 to survive)
What organism & which of its chromosomes did we use to study CTD? Why did we use this organism?
We removed chromosomes from salivary glands in the drisophila
Because these are polytene chromosomes (have a lot of copies)
There is a banding pattern that occurs within the salivary chromosomes (the puffed up bands are associated with genes that are known to be actively transcribed)
What did we do with the salivary chromosomes of the drisophila? (conclusions about phosphorylated/unphosphorylated CTD)
We took the salivary chromosomes and stained them with an antibody that recognizes RNA polymerase II CTD domain when its nonphosphorylated OR its phosphorylated form, you get a very different pattern
The antibody that binds to only the phosphorylated form binds only to the puffed up regions, suggesting that the phosphorylated form of the CTD domain is associated with actively transcribed genes
The antibody that binds only to the unphosphorylated form binds to non-puffed regions / inactive regions
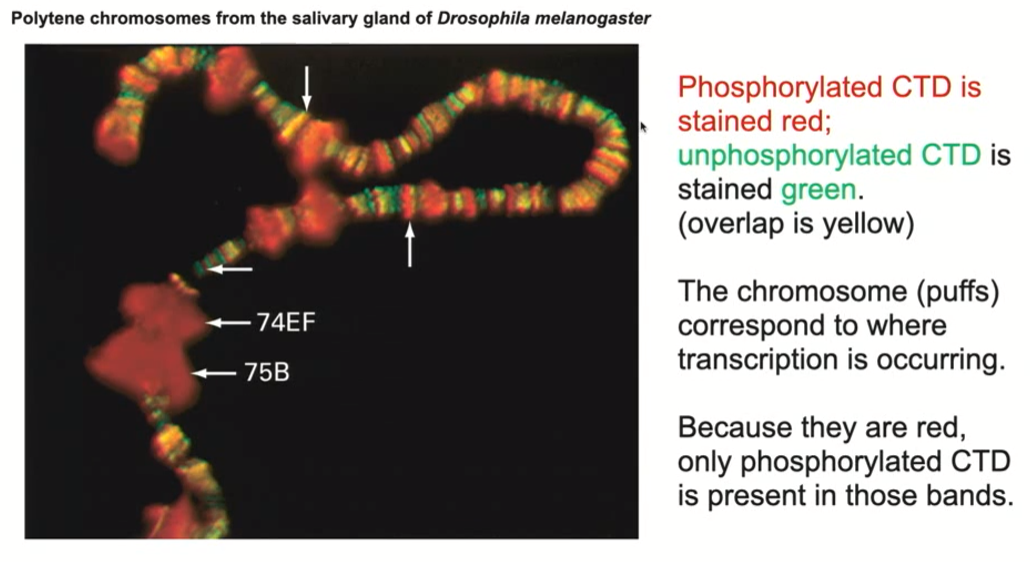
Which form of CTD in the large subunit of RNA polymerase II is associated with active transcription? (FInal conclusion summary
the phosphorylated form of CTD is associated with active transcription
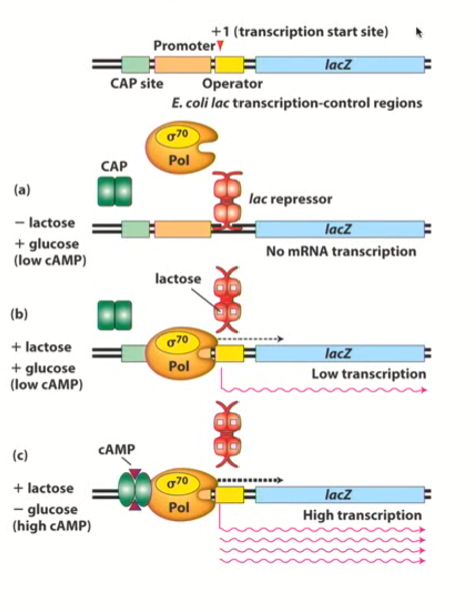
Lac Operon initiation
Turned on by bacterial RNA polymerase II, but it needs help identifying where to bind (what genes need to be activated/the promoter region)
Sigma factors tells RNA polymerase where to bind by recognizing specific sequences in the upstream region (the promoter region)
Proteins are recognizing sequences in the DNA which is instructing them where to interact
There may be additional sequences upstream that enhance the binding of the RNA/sigma factor complex, or even do the opposite and repress it
TATA box initial discovery (+ basic definition)
people noticed that about 35 nucleotides upstream of the transcriptional start site, there was some sort of common sequence that seemed to be present in many genes
This sequence corresponded to a “T-A-T-A-A/T-A-A/T-A/G”
Because of this common “TATA” sequence, these regions were named TATA boxes
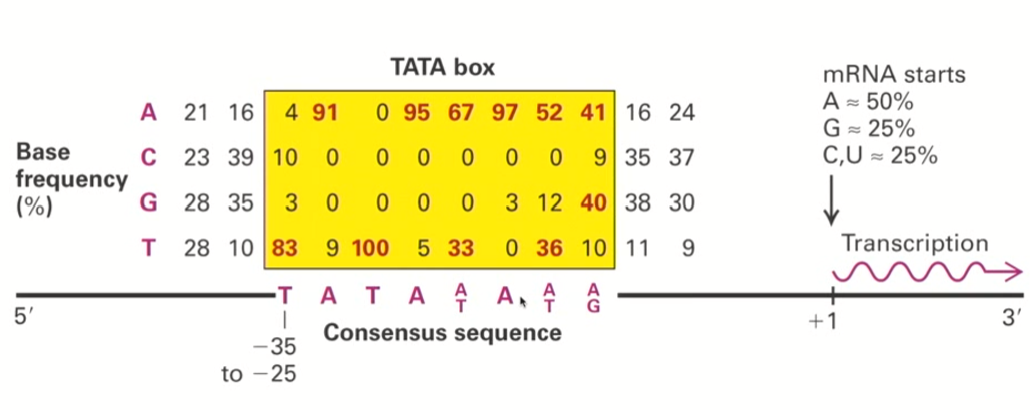
Why did we think TATA boxes were important for transcription?
Because in certain cases, if you eliminated the TATA box in highly expressed genes, they were not expressed well anymore.
This is an example of a regulatory sequence present in the promoter sequence that is critical for the regulation of a downstream target
Other proximal “cis-acting” elements that contribute to transcriptional initiation aside from TATA-boxes
TFIIB response element (BRE)
Initiator Sequence
Downstream Promoter Element
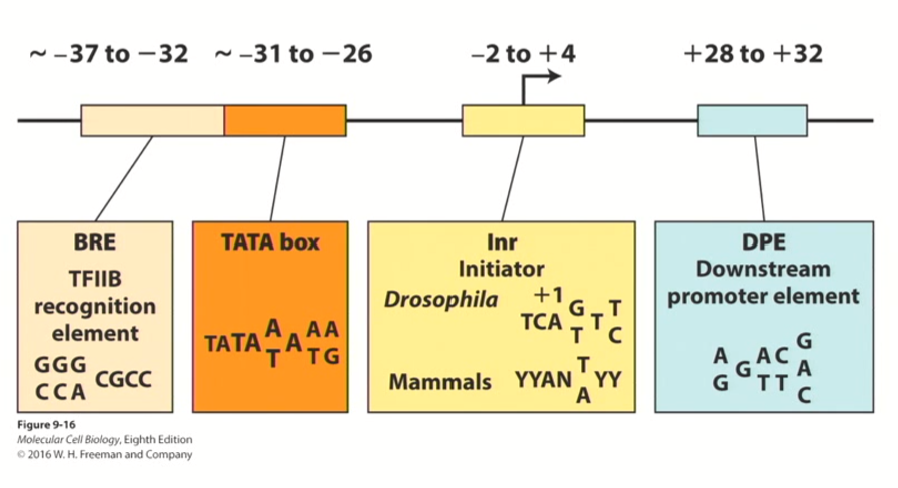
TFIIB response element (BRE)
this is a very important general transcription factor for all genes transcribed using RNA polymerase II (class II genes)
Acts in -37 to -32 region of the promoter
Initiator Sequence
Not as conserved as TATA box or BRE, but seems to contribute to efficiency of transcription in eukaryotic genes
acts in -2 to +4 region of the promoter
Downstream Promoter element
these sequences are not highly conserved, but they do contribute either collectively or individually to the efficiency of transcriptional initiation
Acts in +28 to +32 region of the promoter
What does “cis-acting element” mean?
Sequences that are affecting the transcription of their associated downstream gene (working on the same strand)
Small sequences that provide information to express a downstream gene
cis = same
Proximal promoter elements
loose term to describe these cis-acting elements that will work in the first 200 nucleotides upstream of the transcriptional start site
Proximal = close to transcriptional start site
Depicted by brown rectangles in the diagram
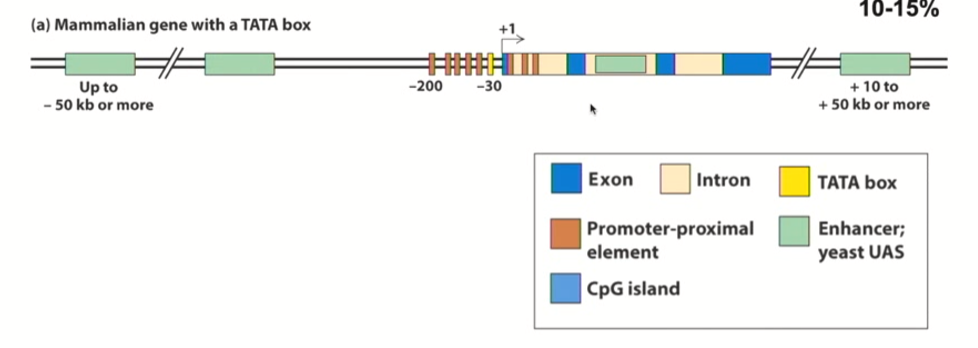
Enhancer region
DNA regulatory elements that are VERY FAR from the genes they are affecting
Can be upstream or downstream

How much of the mammalian gene is made up of TATA-containing promoters (proximal and enhancer)?
These make up 10-15% of the mammalian gene
How have genes evolved? (How do they look today in comparison?)
Do not really have a TATA-box region, have a much more complex structure
Promoters are filled with CPG promoter sequences - they can drive transcription in both directions
How much of the mammalian gene is made up of CPG island promoters?
about 50-75%
What are CpG promoters usually associated with?
Bi-Directional Transcription
Housekeeping genes, not necessarily highly activated
Yeast gene structure
far more simple
Transcriptional start site
Very few introns
obvious TATA-Box
some sort of distant enhancer known as a UAS (upstream activating sequence) that is a hybrid between a proximal promoter and an enhancer
Downstream gene that is regulated

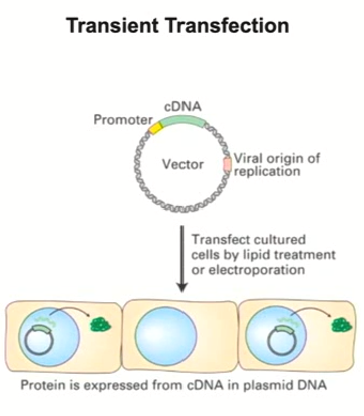
What is recombinant DNA technology used for? (General Idea)
To test how a piece of DNA affects transcription
If you have a specific chunk of DNA (a regulatory element you are interested in), you can test exclusively that regulatory element and how it contributes to the activation of transcription by taking that element and putting it in a specialized plasmid that is important for expression in eukaryotic cells
Most important thing is that you have a reporter gene
What is a reporter gene?
Something that tells you when transcription is on during recombinant DNA technology
Recombinant DNA technology general steps
Clone the regulatory element (promoter/enhancer) in a plasmid vector, which will allow you to express a given reporter gene
Introduce a reporter gene that will tell you when transcription is active
Insert the plasmid into the eukaryotic cells using transfection

What is transfection?
The process that inserts the plasmid into a eukaryotic cell
Can occur through electroporation - a jolt of electricity is applied to the cell which opens the channels and allows DNA to flow in
Can occur through lipid treatment - a specific lipid compound is applied that makes the cell’s membrane permeable to DNA
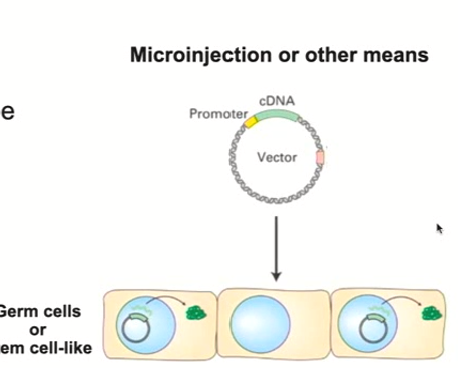
Transgenics general definition
Recombinant DNA technology in live animals/plants
Transgenics process
Uses same type of technology to make specific plasmids (cloning, etc.)
Introduce them into the organisms so that the DNA will go into the germ cells, or stem cell like cells
This propagates the changes you made to future generations
How to identify where regulatory elements are? “Quick and dirty” method (Pt 1 - until transfection)
Carry out a 5’ deletion series
Take the entire section that you know is sufficient for the transcription for that gene (TTR) and put it into an expression vector upstream of a given reporter gene
In the first example (taking 4kb upstream region) which you know is sufficient to activate the transcription, you put this into an expression vector (think of this as your control - contains all sequences
Black dot refers to the sequences around the transcriptional start site
Then, you can do PCR reactions to make smaller and smaller segments, where you are removing some of the 5’ information
All of the PCR products you generated can be put into a specific plasmid that contains the same reporter gene in each case
You ligate the reporter gene in so that you have 5 different plasmids
transfect each of the 5 expression vectors into a host cell

How to identify where regulatory elements are? “Quick and dirty” method (Pt 2 - transfection and beyond)
Expression vector will be taken up, and, when transcription starts to happen, that cell will make the mRNA that corresponds to the reporter, that mRNA will be translated and then give rise to whatever protein the reporter encodes for
The amount of product made should correlate with how well that mRNA is produced
Then you can quantify that particular reporter gene product
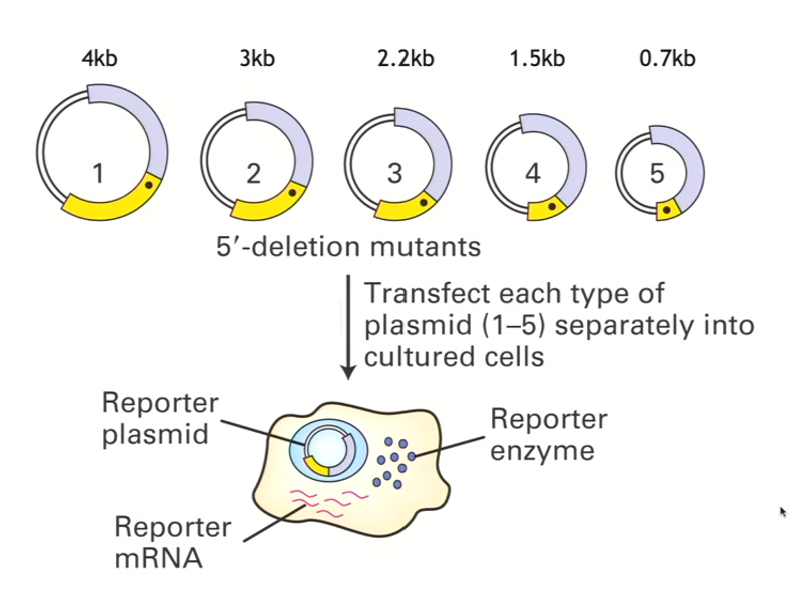
Quantifying the result of a 5’ deletion series
compare to longest segment/control which we know contains all regulatory elements (in this case, 4kb)
we can see that 3kb also has great reporter-gene expression
2.2kb has less reporter gene expression, so an element that activates transcription must lie between 2.2 and 3kb
and so on! process of elimination
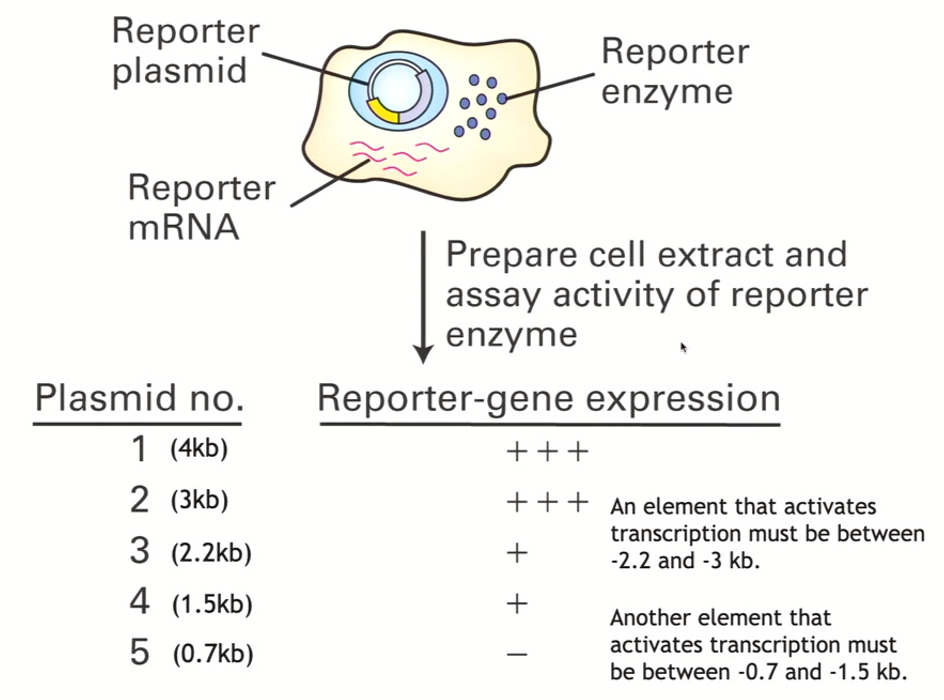
Why is the transfection method known as the quick and dirty method?
Because you are not respecting the size of that upstream region, you are cutting out and making small segments
in other words, not as accurate
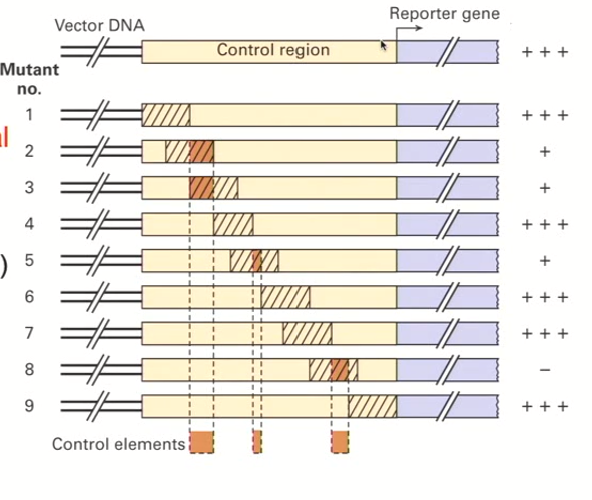
Linker-scanning mutational analysis process + why we use it
You remove segments of the regulatory region that overlap just a little bit (very precise)
This keeps the promoter’s length constant
Each of the variants (removed regions) are tested for a reporter gene activity
When a segment results in a decrease in reporter expression, this means that the variant region contains important transcriptional elements
We can identify critical control elements that are present in very large sequences
Examples of effective reporter genes
Beta-galactosidase
Green fluorescent protein (GFP)
Thymidine kinase (tk)
luciferase (luc)
chloramphenicol acetyltransferase (CAT)
Enhancer’s critical role in development
There are specific DNA binding proteins that interact with enhancers
These DNA binding proteins are somehow critical for impinging on the transcriptional efficiency of a given target gene
They’ll confirm tissue specific information to ensure that only one tissue will express a particular gene
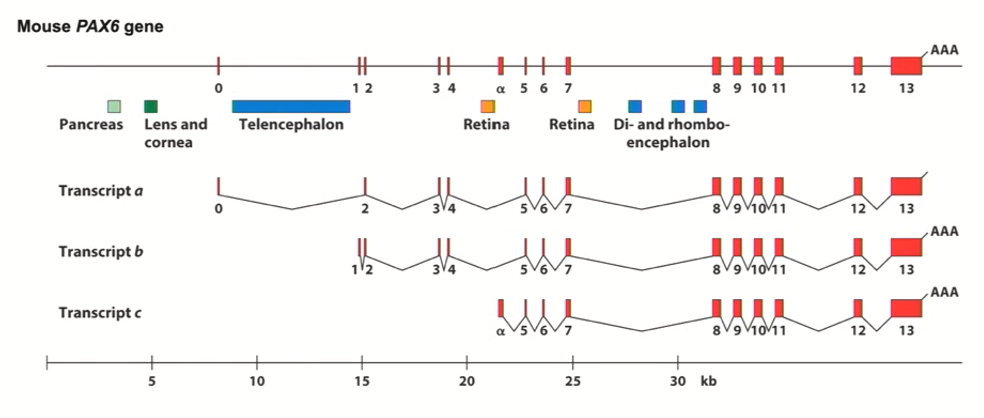
PAX6 Gene
paired box transcription factor
It is involved specifically in eye development/other neurological features
if you take the PAX6 homolog (known as eyeless in flies) and express it in any cell within the fly, the legs will start to create an eye
Master regulator of eye development
It has 3 promoters
It has many enhancers that drive expression in different tissues (such as retina, lens, etc.)
Humans and mouse have similar PAX6 gene (it is highly conserved)
Cell 1 gene for analyzing crucial enhancers + transgenic mouse
large, critical gene involved in limb development
If we compare the sequence across organisms, it shows most regions changed with evolution. However, some small regions stayed highly conserved
These are likely crucial enhancers
If we clone one of these crucial/conserved regions downstream of Cell 1 and use an effective reporter (beta-galactosidase), we can create a transgenic mouse (beta-galactosidase will stain the limbs blue if Cell1 is expressed). Very evident blue stains ended up appearing in the developing limbs of the mouse, so…
The single conserved enhancer is enough to drive Cell1 expression in developing limbs
How does chromosomal structure help enhancer expression despite enhancers being so far away?
chromosomes are not linear, they are all folded up
Chromatin looping brings enhancer and promoter regions closer together
This allows for easier communication between important regions
