P1 Energy
1/128
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
129 Terms
Energy stores
magnetic
internal (thermal)
chemical
kinetic
electrostatic
elastic potential
gravitational potential
nuclear
magnetic energy
The energy stored when repelling poles have been pushed closer together or when attracting poles have been pulled further apart.
magnetic energy - examples
fridge magnets
compasses
maglev trains (use magnetic levitation)
Internal energy
The total kinetic and potential (kinetic) energy in particles in an object.
Internal energy - examples
human bodies
hot items
all items as every item’s particles vibrate - even ice particles vibrate just slower
Chemical energy
Energy stored in chemical bonds, such as those between molecules.
Chemical energy - examples
foods
muscles
electrical cells
Kinetic energy
The energy of a moving object.
Kinetic energy - examples
runners
buses
comets
Electrostatic energy
The energy stored when repelling charges have been moved closer together or when attracting charges have been pulled further apart.
Electrostatic energy - examples
thunderclouds
van de graff generators
elastic potential
The energy stored when an object is stretched or squashed.
Elastic potential - examples
drawn catapults
compressed springs
inflated balloons
Gravitational potential
The energy of an object at height.
Gravitational energy - examples
aeroplanes
kits
mugs on a table
Nuclear energy
The energy stored in the nucleus of an atom.
Nuclear energy - examples
uranium nuclear power
nuclear reactors
How long can energy remain in the same store?
sometimes millions and millions of years, or sometimes just for a fraction of a second.
Whenever a system changes…
…there is a change in the way some or all of the energy is stored.
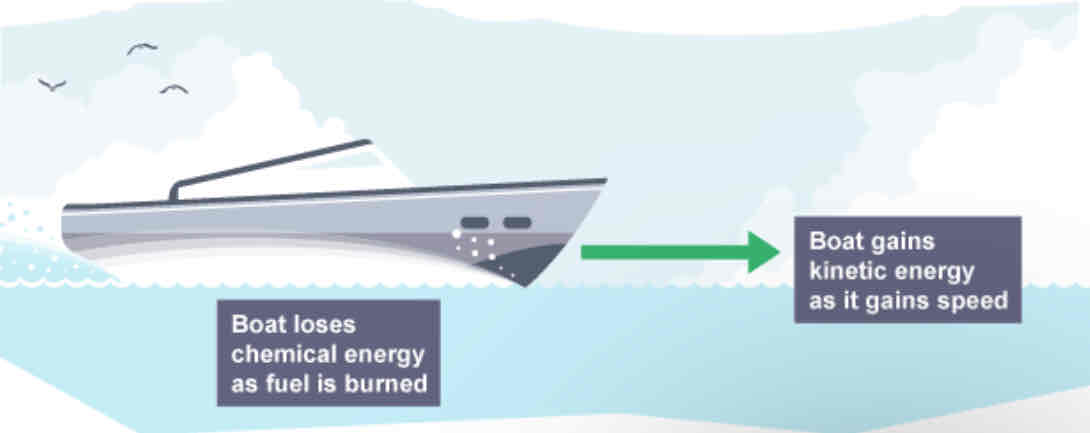
Energy transfers in a boat
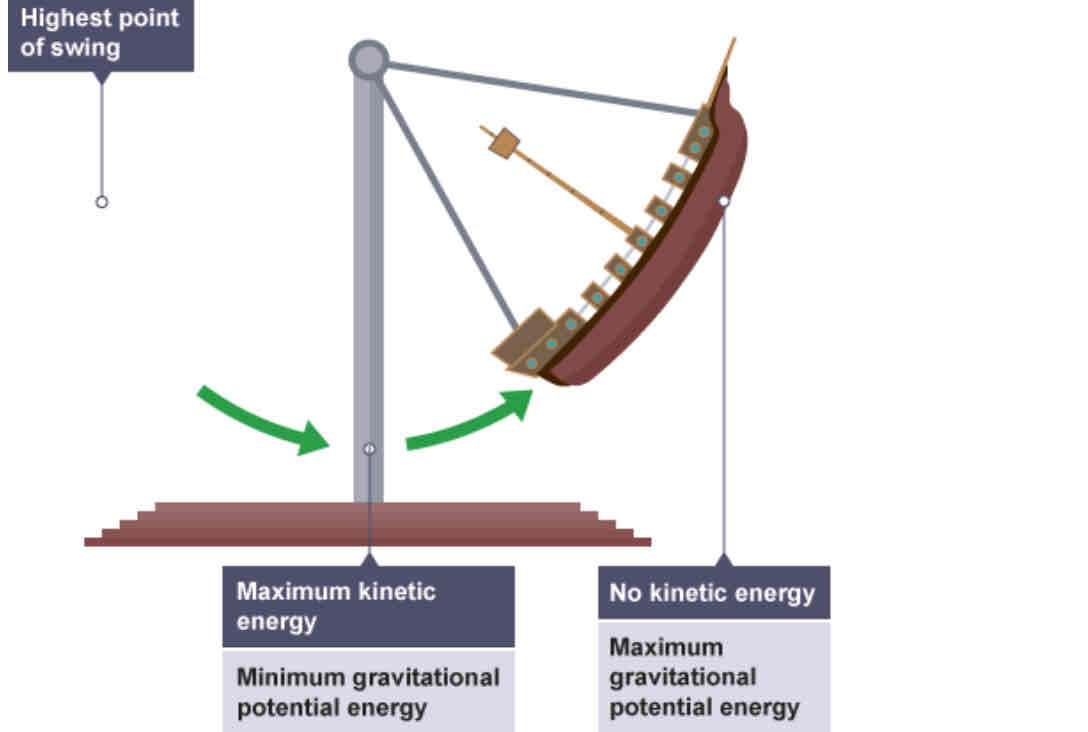
Energy transfers in a swinging pirate ship
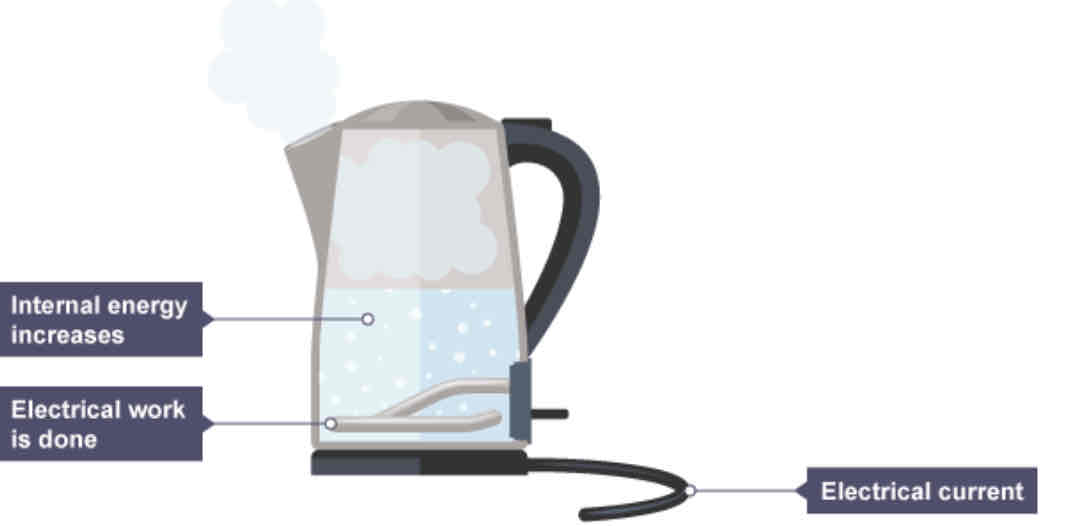
Energy transfers in a boiling electric kettle
What are the 4 ways energy can be transferred?
mechanical work
electrical work
heating
radiation
mechanical energy transfer
A force moving an object through a distance
electrical energy transfer
charges moving due to a potential difference
energy transfer by heat
due to temperature difference caused electrically or by chemical reaction
energy transfer by radiation
energy transferred as a wave, eg. light and infrared emitted from the sun
What diagrams are used to show energy transfers?
Sankey Diagrams and transfer diagrams
Sankey diagrams - explanation
Starts off as one arrow that splits into 2 or more points. This shows how all of the energy in a system is transferred. The width of the arrow is drawn to scale to show the amount of energy.
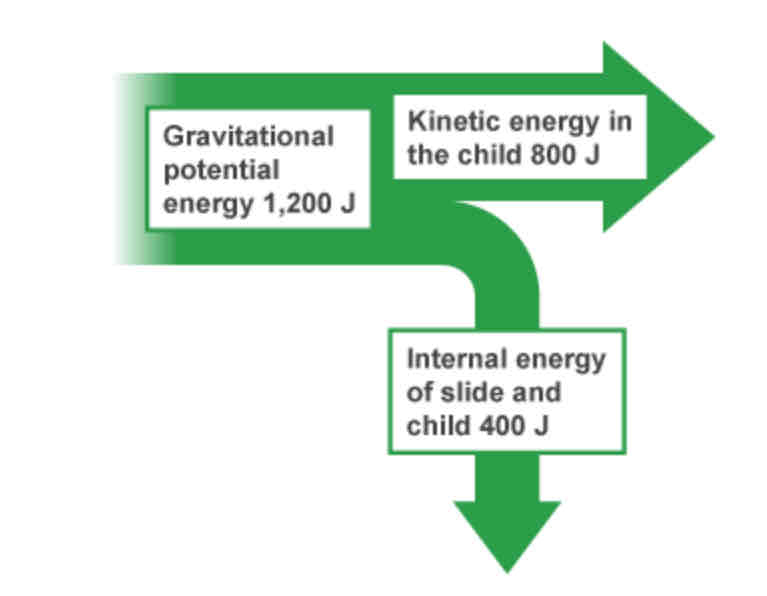
When are Sankey diagrams useful?
When the amounts of energy in each of the energy sources is known.
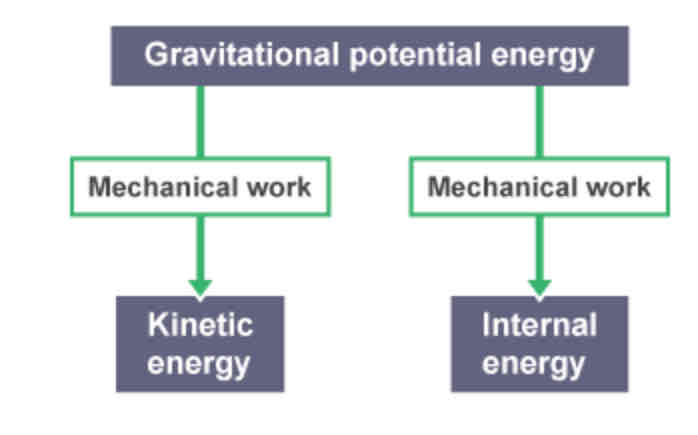
Transfer diagrams - explanation
Boxes → show the energy stores
Arrows → show the energy transfers
dissipated
The spreading out and transfer of energy stores into less useful forms, eg. Causing surroundings to heat up.
Dissipated energy is often referred to as…
‘Wasted’, as it is not transferred to a useful output.
What are examples of dissipated energy? How can this be prevented?
Electrical cables heating up → wrap wires up in insulator
When 2 surfaces rub together, increasing their internal energy → add lubricant so less heat lost by friction
radio/speakers waste energy as infrared radiation
tumble dryer wastes energy as sound waves
The conservation of energy
Energy cannot be created or destroyed, only tranferred
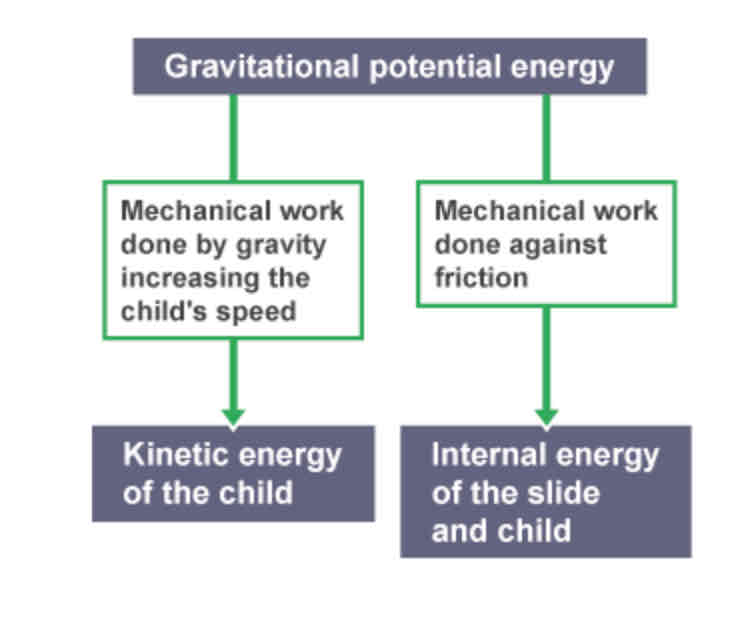
Example of conservation of energy - skydiver
Skydiver begins to lose gravitational potential energy as he falls out of the plane, but gains kinetic energy as his speed increases. This transfer is mechanical. Some of the energy is dissipated to the air particles as skydiver pushes against them, so they gain internal energy.
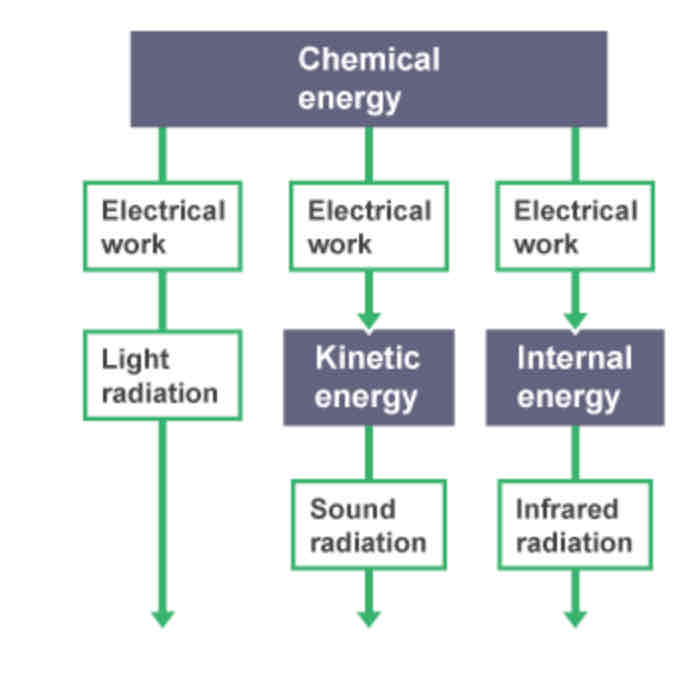
Example of conservation of energy - Smartphones
Contain a battery storing chemical energy. When it is in use, electrical work is done and current flows, so the battery’s chemical energy is transferred in many ways (light, sound, …).
Light on screen is emitted as light radiation, and sound waves are produced by speaker vibrating back and forth.
Smartphones also heat up, transferring energy into internal energy which is stored in the atoms of the smartphone’s conductors which emit infrared radiation.
Kinetic energy equation
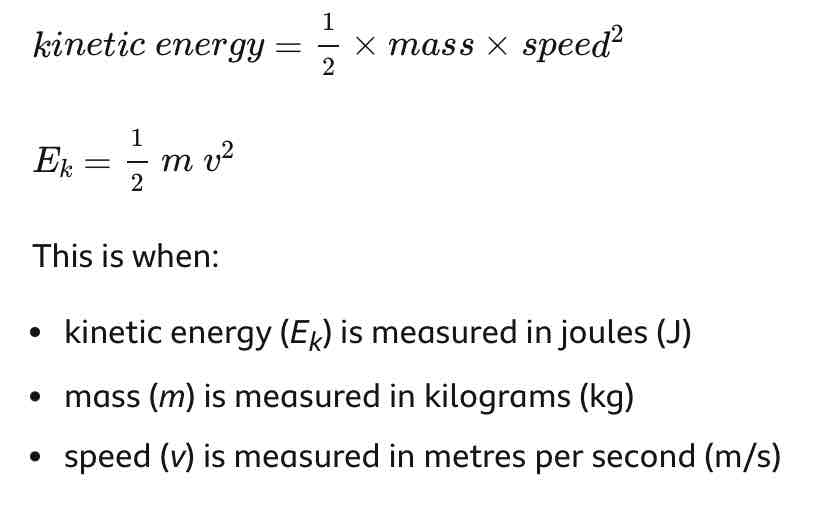
Elastic potential energy
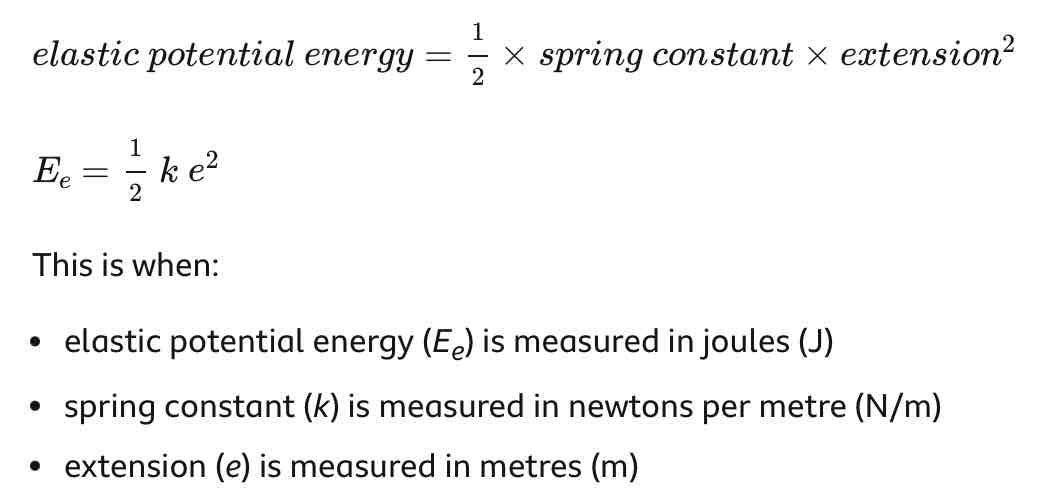
Gravitational potential energy equation

Work Done Explanation
It is a measure of the energy transfer when a force (F) moves an object through a distance. This means…
energy transferred = work done
…so they are both measured in Joules.
What does the amount of work done depend on?
The size of the force acting on the object
The distance through which the force causes the body to move in the direction of a force
Work done equation
force/distance = work done

When work is done on an object…
…energy is transferred.
Power explanation
The rate at which energy is transferred. The more powerful a device is, the more energy it will transfer each second.
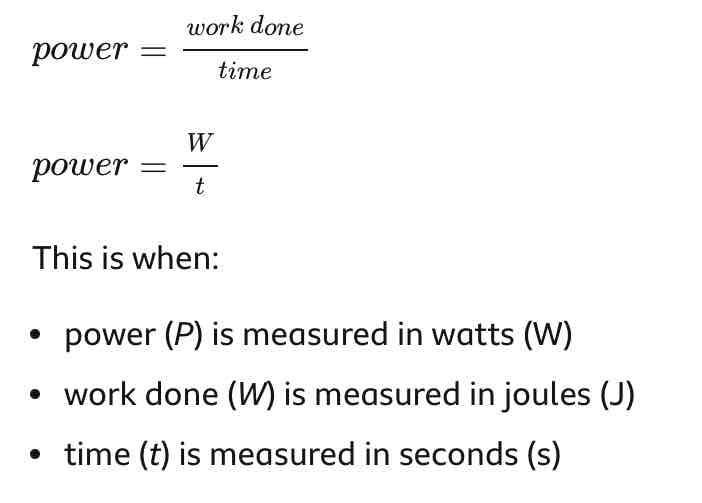
Power equation
work done/time = power
1 Watt is equal to…
…1 Joule per second (Power).
What are devices designed to do?
Waste as little energy as possible. As much of the input energy as possible should be transferred into useful energy stores.
Efficieny explanation
How good a device is at transferring energy input into useful energy output.
A very efficient device will…
…waste very little of its input energy.
A very inefficient device will…
…waste most of its input energy.
In other words, what is the efficiency of a device?
The proportion of the energy supplied that is transferred in useful ways. Can be calculated as a decimal or a percentage.
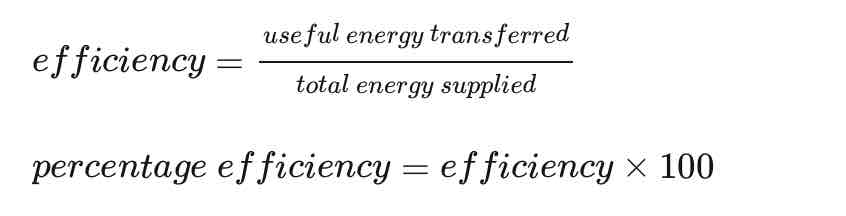
Efficiency equation
Both useful and total energy transfer is measured in Joules.
What is the link between efficiency and power?
Power is equal to the useful energy transferred per second.
Efficiency - alternative equation
Both useful and total power output are measured in Watts.
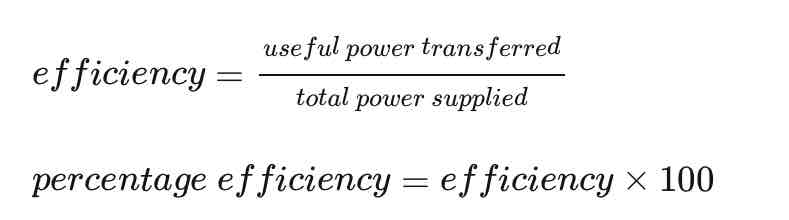
It is/is not possible to have an efficiency greater than 1 or 100%.
Is not
This would mean that more energy is being transferred than being supplied, which would mean that the energy is being created. This would break the law of conservation.
What are examples of electrical appliances which transfer energy?
Electric kettle
Hair dryer
Light bulb
TV
Electric kettle - Useful and Wasted energy
Energy that heats water.
Internal energy heating kettle and infrared radiation transferred to surroundings.
Hair dryer - Useful and Wasted energy
Internal energy heating air and kinetic energy of fan blowing the air
Sound radiation, internal energy heating the hairdryer, infrared radiation transferred to the surroundings.
How is energy transmitted from space to space?
conduction
convection
radiation
Conductor
A material that allows internal (thermal) energy to be transmitted through it easily.
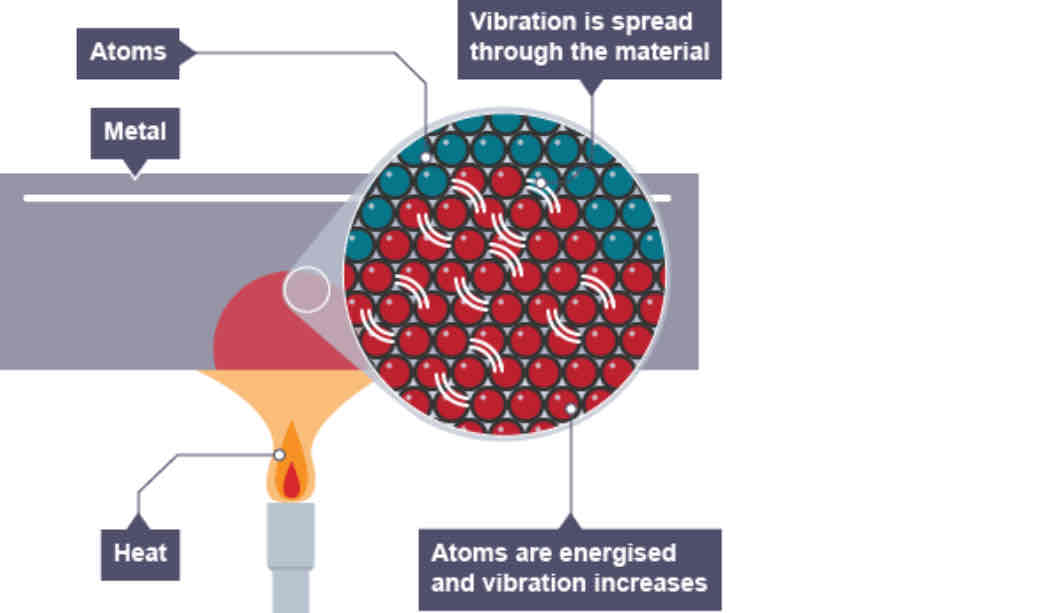
What happens when one end of a metal rod is put into a fire?
The energy from the flame makes the ions in the rod vibrate faster. Since the ions in the solid metal are close together, this increased vibration menas that they collide with neighbouring ions more frequently. Energy is passed on through the metal by these collisions, transmitting energy. More frequent collisions increase the rate of transfer.
Insulator
A material which does not allow the easy flow energy.
Examples of insulators
cushion on a chair
How can you compare the conductivity of materials?
By examining the time taken to transmit energy through them.
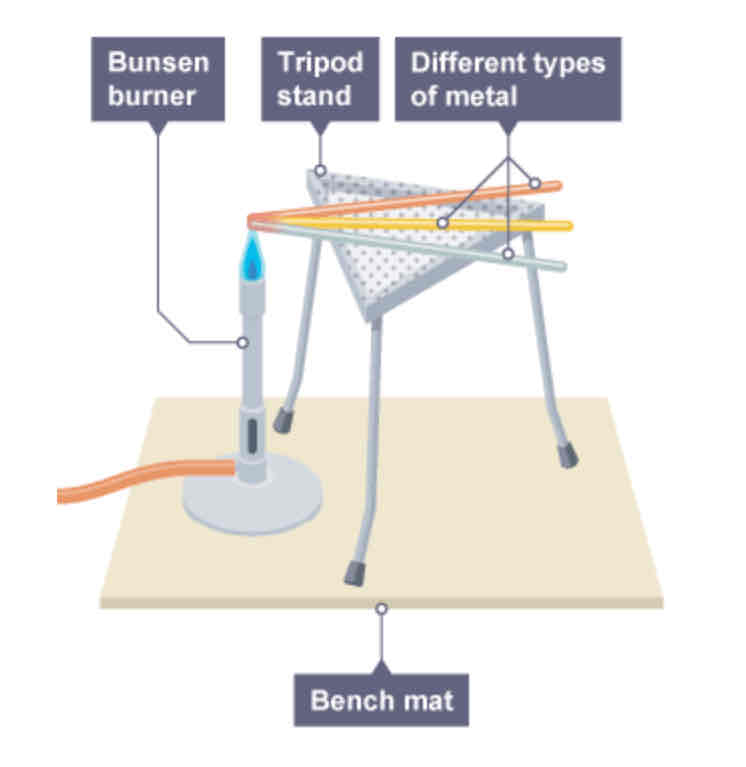
What is an example of comparing conductivities?
Make a fan of rods made of different materials, and heat it at one end with the same flame, whichever rod gets hottest first at the other end is the best conductor, and is said to have thermal conductivity.
Thermal conductivity
A measure of how well a material conducts energy when it is heated.
What are thermal conductivities measured in?
W/m/°C
What is the thermal conductivity of air?
0.024 W/m/°C
Cubic metre
Dimensions 1m x 1m x 1m
How can we insulate homes?
By using materials that are poor conductors - brick, wood, plastic, glass - as energy is not able to be transferred to the home’s surroundings easily.
What are the practicals in this topic?
Investigating methods of insulation - materials
Investigating methods of insulation - thickness
Measuring specific heat capacity
What happens when materials are heated?
The molecules gain kinetic energy and start moving faster. The material gets hotter.
Temperature
A measure of the average kinetic energy of the molecules.
Materials require the same / different amounts of energy to change temperature.
Different
The amount of energy a material needs to change temperature depends on…
the mass of the material
the substance of the material (specific heat capacity)
the desired temperature change
Materials with low/high specific heat capacities will warm up and cool down the fastest. This is because…
Low
This is because it doesn’t take up too much energy to change its temperature.
Specific Heat Capacity
The energy required to raise 1kg of a material by 1°C.
Specific Heat Capacity Equation
mass x SHC x temp change = energy
Specific Latent Heat of Fusion
The energy needed to change the state of 1kg of the substance from a solid to a liquid, at its melting point
Specific Latent Heat of Vapourisation
the energy required to change 1kg of a substance from liquid to gas at its boiling point
Specific Latent Heat Equation
SLH x mass = energy
Specific Heat Capacity of water
4,200
Specific Heat Capacity of brick
840 J/kg°C
energy resources
Systems that can store and supply large amounts of energy.
What are the major energy resources available to produce electricity? (9)
fossil fuels
nuclear fuel
bio-fuel
wind
hydroelectricity
geothermal
tidal
water waves
the sun
Fossil fuels
Natural, finite fuel formed from the remains of living organisms, eg. oil, coal, natural gas.
Nuclear fuel
Radioactive materials, usually uranium or plutonium, used in nuclear reactors.
Hydroelectricity
Electricity generated by the movement of water.
Geothermal
Energy from the heat of the earth.
Where does all the energy on the earth come from?
Ultimately it is from the sun, but it has been stored as different energy resources.
Where is energy needed?
Homes
Public service
Factories and farms
Transport
Why is energy needed in homes?
for cooking, heating and running appliances
Why is energy needed in public services?
running machinery and warming rooms
examples include schools and hospitals
Why is energy needed for transport?
buses, trains, cars and boats all need a fuel source and some trains and trams connect to an electricity supply
Why might producing and distributing electricity not be good?
Releasing some energy stores can cause damages to the environment by causing pollution and releasing waste products.
What is the effect of burning fossil fuels?
Releases carbon dioxide, adding to the greenhouse effect, and sulphur dioxide which causes acid rain.
What happened during the Industrial Revolution?
Advances in automation and transport caused a significant increase in the amount of fossil held extracted and burnt.
What happened in the 20th century?
electricity used to distribute energy. This powered a range of devices eg. lighting, heating, computing technologies and operating machinery.
How does the demand for energy vary?
Varies with the…
Time of day
Time of year