Honors Chemistry: Gas law vocabulary and formulas
1/28
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
relates volume and pressure at a constant temperature
Boyles law
Volume and pressure inversely related
Boyles law
formula: P1V1 = P2V2
Boyles law
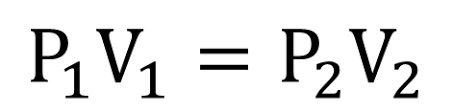
if you push a bottle and it gets crushed…this law is….
Boyles law
relates temperature and volume at a constant pressure.
Charles law
temperature and volume are directly related
Charles law
formula: V1/T1 = V2/T2
Charles law
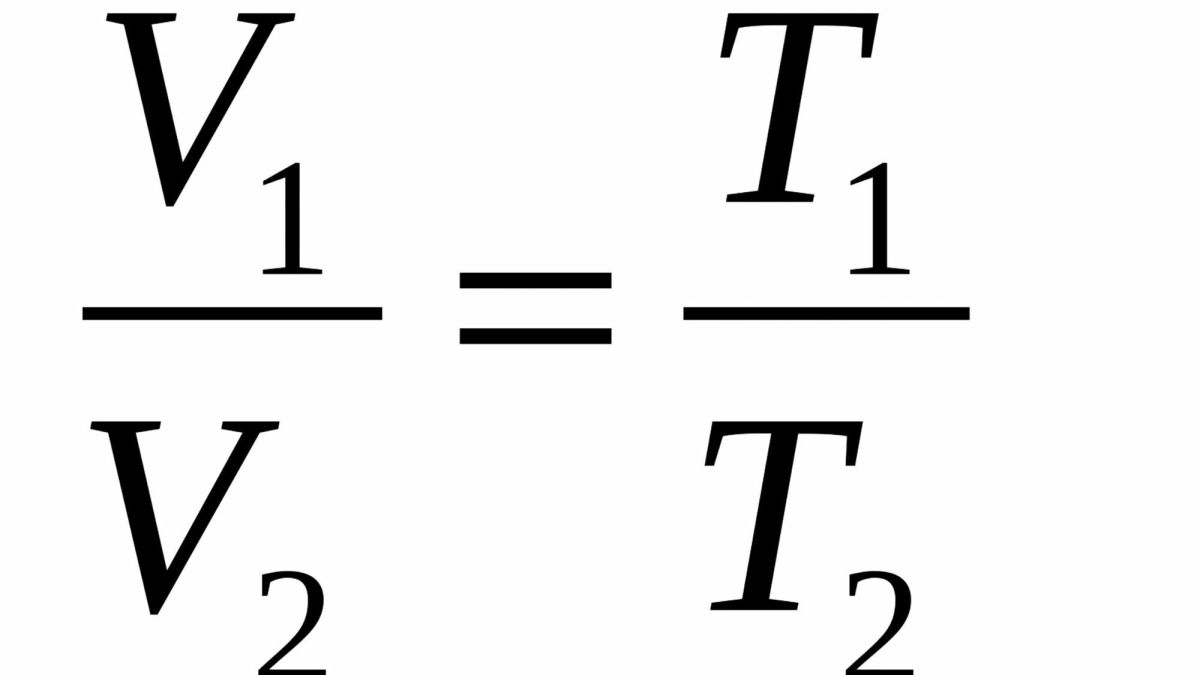
a hot air ballon is what law…
Charles law
relates pressure and temperature at a constant volume
Gay Lussacs law
pressure and temperature are directly related
Gay Lussacs law
formula: P1/T1 = P2/T2
Gay Lussacs law
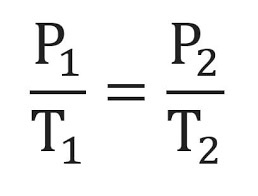
Hairspray in a fire that causes an explosion is what law….
Gay Lussacs law
pressure, temperature, volume, # of particles (moles) are all interrelated.
Ideal gas law
formula: Pv = nRT
Ideal gas law
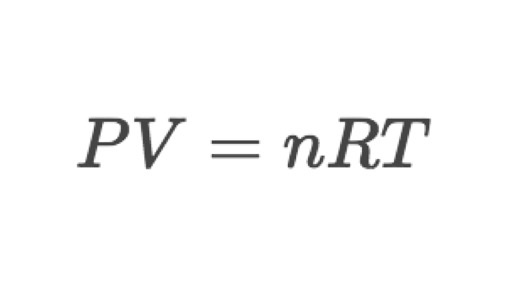
In a refrigerator, the coolant gas is compressed which means the volume of gas is decreased that eventually increases the temperature…this laws is….
Ideal gas law
Each gas independently creates pressure
Dalton law of partial pressure
displacement technique to find pressure which often uses water
Dalton law of partial pressure
formula: Pt = P1 + P2 + P3……
Dalton law of partial pressure
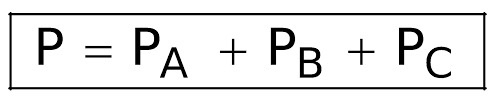
a Scuba tank. The air in a scuba tank is carefully regulated to a specific mole ratio. That means that each of those gases in that tank will have their own partial pressure…this law is…..
Dalton law of partial pressure
if you have more moles, takes up more space.
Avogadros law
relates volume and moles directly
Avogadros law
formula: V1/N1 = V2/N2
Avogadros law
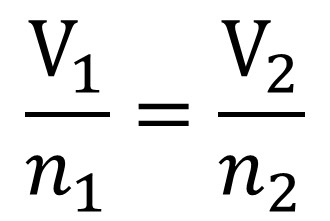
The way car tires loses air. When the air that was trapped in the tire gets out, the amount of air in the tire goes down….this law is….
Avogadros law
pressure, volume, temperature all directly related
Combined gas law
formula: P1V1/T1 = P2V2/T2
Combined gas law
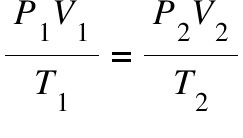
As the temperature of the refrigerated balloon decreases, the volume of the gas inside the balloon also decreases…this law is….
Combined gas law
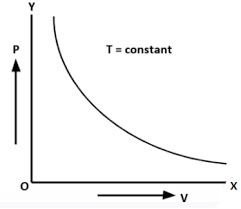
Boyles law
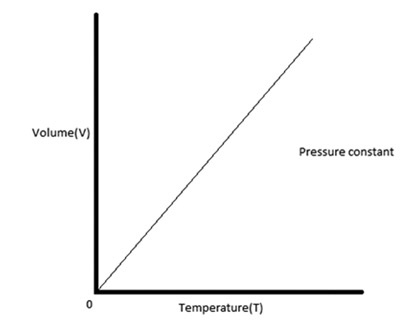
Charles law
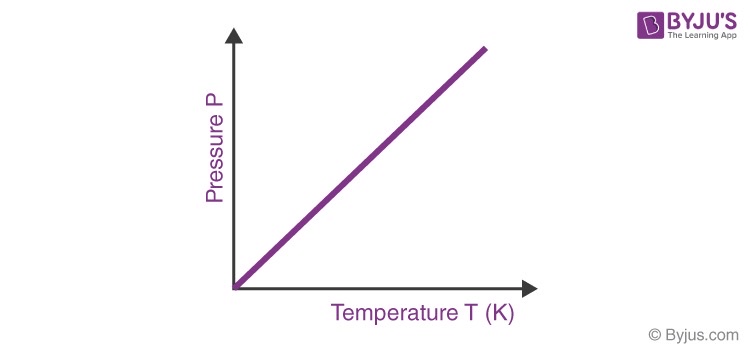
Gay Lussacs Law