MCAT Organic Chemistry - COMPLETE
1/367
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
368 Terms
International Union of Pure and Applied Chemistry (IUPAC)
simplies chemical naming; unambiguous relationship between the name and structure of a compound; no two compounds have the same name
parent chain
Longest Carbon Chain Containing the Highest-Order Functional Group
Numbering
highest priority functional group/substituted carbons with the lowest number; assign substituents the number of carbon they are attached to
heteroatoms
atoms besides carbon and hydrogen, like oxygen, nitrogen, phosphorus, or halogens
Substituents
functional groups that are not part of the parent chain; assign the number of carbon they are attached to; numeric and hyphenated prefixes are ignored while alphabetising
carbon chain substituents
end with -yl
n- means normal
multiple - prefixes di-, tri-, tetra-, etc. directly before substituents
Alkanes
simple hydrocarbon molecules with the formula CnH(2n+2); saturated - only single bonds
name is the Greek root describing the number of carbons followed by –ane
Alkyl halides
indicated by a prefix: fluoro–, chloro–, bromo–, or iodo–; number the carbon
alkenes
simple hydrocarbon molecules with the formula CnH2n; unsaturated - at least one double bond
ends with -ene; number starting carbon of bond before parent or before suffix; number of multiple bonds prefix added to suffix
alkynes
simple hydrocarbon molecules with the formula CnH(2n-2); unsaturated - at least one triple bond
end with -yne; number starting carbon of bond before parent or before suffix; number of multiple bonds prefix added to suffix
Alcohol/hydroxyl group
―OH
ends with alykl parent suffix - e + ol; higher priority than alkyl chains - number each before suffix; if no priority, hydroxy- substituent; often have common names - name of alkyl group + alcohol
diol/glycol
alcohol with two hydroxyl groups
entire hydrocarbon + -diol; number each hydroxyl group
geminal diols/hydrates
diols with hydroxyl groups on the same carbon
not commonly seen because they spontaneously dehydrate to produce carbonyl compounds
vicinal diols
diols with hydroxyl groups on adjacent carbon
carbonyl group
―C=O
chain-terminating substituents
appear in the end of carbon chains
Aldehyde
carbonyl group found at the end of the carbon chain
parent alkane - e + al; do not include number if on carbon 1; if not priority, oxo- prefix
common names: formaldehyde; acetaldehyde; propionaldehyde
aldose sugars
Ketone
carbonyl group somewhere in the middle of the carbon chain
parent alkane - e + al; always include number; also alkyl groups in alphabetical order + ketone, if not priority, oxo-/keto- prefix
common names; acetone (2-propanone; smallest ketone)
ketose sugars
carbon names adjacent to carbonyl
adjacent to the
carbonyl carbon is indicated by alpha (α), then beta (β), gamma (γ), and delta (δ)
both sides of ketone
Carboxylic (organic) acids/carboxyl group
contain both a carbonyl group (C=O) and a hydroxyl group (―OH) on a terminal carbon; also written (COOH); highest priority functional group
parent alkane - e + -oic acid
common names: formic acid, acetic acid, propionic acid
Esters
COOR; hydroxyl group is replaced with an alkoxy group
name of parent acid - -oic acid + -oate; if not priority, alkoxycarbonyl- prefix
aloxy group
-OR, where R is a hydrocarbon chain
amide
CONR, hydroxyl group is replaced by an amino group; complex—the amino nitrogen can be bonded to zero, one, or two alkyl groups
name of parent acid - -oic acid + -amide; substituents attached to nitrogen are labeled with N-; if not priority; carbamoyl-/amido- prefix
amino group
a substituent group containing nitrogen
anhydride
In the formation of an anhydride from two carboxylic acid molecules, one water molecule is removed; symmetric if 2 same acid, asymmetric if 2 different acids; cyclic if intramolecular reaction of dicarboxylic acid
name of parent acid - acid + anhydride
Order of priority
Carboxylic acid > anhydride > ester > amide > aldehyde > ketone > alcohol > alkene or alkyne > alkane
isomers
compounds with the same molecular formula but different structures; same molecular weights
relative term
structural/constitutional isomers
do not have the same connectivity; least similar; vary in physical and chemical properties
stereoisomers
have the same connectivity/structural backbone
For any molecule with n chiral centers, there are 2n possible stereoisomers
conformational isomers/conformers
type of stereoisomer, do not require bond breaking to interconvert, differ in rotation around single bonds; interconversion barrier may be easy to overcome at room temperature but not at low temps.
configurational isomer
type of stereoisomer, requires bond breaking to interconvert
diastereomers
type of configurational isomer, molecules with 2+ stereogenic centers that differ at some, but not all, of them; any stereoisomer that is NOT an enantiomer
optically active
enantiomers
type of configurational isomer, nonsuperimposable mirror images
optically active
cis-trans isomers (formerly geometric)
type of diastereomers, differ in arrangement around an immovable bond
physical properties
observable with no change in composition of matter
ex. melting point, boiling point, solubility, odor, color, density.
chemical properties
reactivity of molecule, resulting in change in composition; generally attributable to functional groups in the molecule.
Newman projection
molecule is visualized along a line extending through a carbon–carbon bond axis
staggered conformation
no overlap of atoms along the line of sight
anti conformation
type of staggered conformation; the two largest groups are antiperiplanar (in the same plane, but on opposite sides) to each other; lowest energy state
gauche conformation
type of staggered conformation; when the two largest groups are 60° apart
(also means unsophisticated or awkward)
eclipsed conformation
overlap of atoms along the line of sight; the two largest groups are 120° apart or on top of each other
totally eclipsed conformation
two largest groups directly overlap each other with 0° separation, synperiplanar (in the same plane, on the same side) ; highest energy state
ring strain
a type of instability that exists when bonds in a molecule form angles that are abnormal
arises from three factors: angle strain, torsional strain, and nonbonded/steric strain
Angle strain
when bond angles deviate from their ideal values by being stretched or compressed
Torsional strain
when cyclic molecules must assume conformations that have eclipsed or gauche interactions
Nonbonded/steric strain/van der Waals repulsion
when nonadjacent atoms or groups compete for the same space
flagpole interactions
steric interactions that occur between substituents attached to adjacent carbon atoms in cyclic organic compounds
axial equatorial orientations alternate around the ring
ideal cyclobutane conformation
puckered
ideal cyclopentane conformation
envelope
cyclohexane conformations
chair (most stable)
boat
twist/skew-boat
axial
substituents that are perpendicular to the plane of the ring; sticking up/down
equatorial
parallel to the plane of the ring; sticking out
chair flip
one chair form is converted to another; all axial and equatorial groups switch
bulkiest groups favor equatorial position to reduce flagpole interactions
half-chair conformation
half-chair, half-planar cyclohexane, highest energy level
cis ring
largest groups on same side (up/down) of the ring
trans ring
largest groups on opposite side (up/down) of the ring
optical isomers
another term for configurational isomers due to the fact they can rotate polarised light
chiral
mirror image cannot be superimposed on the original object; molecule lacks an internal plane of symmetry
from Greek word for hand
achiral
mirror image can be superimposed on the original object; molecule has at least one internal plane of symmetry
chiral center
carbon with four different substituents
optical activity
rotation of plane-polarized light by a chiral molecule
specific rotation
the unique angle an optically active compounds rotates polarised light
[α] = αobs/cl
where [α] is specific rotation in degrees, αobs is the observed rotation in degrees, c is the concentration in g/mL, and l is the path length in dm
dextrorotary (d-/(+))
compound that rotates the plane of polarised light to the right/clockwise
levorotatory (l-/(−))
compound that rotates the plane of polarised light to the left/counterclockwise
racemic mixture
when dextrorotary and levorotary enantiomers are present in equal concentration; rotations cancel and no optical activity is observed
can be separated by reacting with another compound’s enantiomer → makes diastereomers with different physical properties
cis isomer
simple substituents over a double bond on same side
trans isomer
simple substituents over a double bond on opposite sides
meso compound
a molecule with chiral centers that has an internal plane of symmetry
not optically active
configuration
spatial arrangement of atoms/groups in a molecule
relative configuration
configuration in relation to another chiral molecule
absolute conformation
exact spatial arrangement of atoms/groups in a molecule, independent of other molecules
Cahn–Ingold–Prelog priority rules
priority is assigned based on the atom bonded to the double-bonded carbons: the higher the atomic number, the higher the priority. If the atomic numbers are equal, priority is determined by the next atoms outward; again, whichever group contains the atom with the highest atomic number is given top priority. If a tie remains, the atoms in this group are compared one-by-one in descending atomic number order until the tie is broken.
E/Z nomenclature
compounds with polysubstituted double bonds
Z (zusammen) - together
E (entgegen) - opposite
R/S forms
used for chiral (stereogenic) centers in molecules
assign priority
Arrange in space so lowest priority is in the back/invert the stereochemistry (remember to switch assignment at end)
Draw a circle number substituents in numerical order
Assign R/S
Write the name
R configuration
rectus/right, clockwise from high to low priority
S configuration
sinister/left, counterclockwise from high to low priority
Fischer projection
horizontal lines = wedges, vertical lines = dashes
determine order of substituents and direction - designation is opposite
Swap the lowest priority group onto vertical axis, then switch the other two - designation is same
principal quantum number, n
corresponds to the energy level of a given electron in an atom and is essentially a measure of size; the smaller the number, the closer the shell is to the nucleus, and the lower its energy; 1 to ∞
azimuthal quantum number, l
corresponds to subshells; ranges from 0 to n−1; 0, 1, 2, and 3 correspond to the s, p, d, and f subshells; energy increases as the azimuthal quantum number increases
magnetic quantum number, ml
corresponds to orbitals; ranges from −l to +l
orbital
describes the probability of finding an electron in a given region of space
s-orbital
spherical and symmetrical, centered around the nucleus

p-orbital
composed of two lobes located symmetrically about the nucleus and contains a node at the nucleus; ‘dumbbell’; three different orientations, along the x-, y-, or z-axis.

node
an area where the probability of finding an electron is zero
d-orbital
composed of four symmetrical lobes, the fifth looks like a donut wrapped around the center of a p-orbital, and contains two nodes; rare in org. chem.
spin quantum number, ms
correspond sto electrons; ±½
molecular orbitals
two atomic orbitals combine, obtained mathematically by adding or subtracting the wave functions of the atomic orbitals
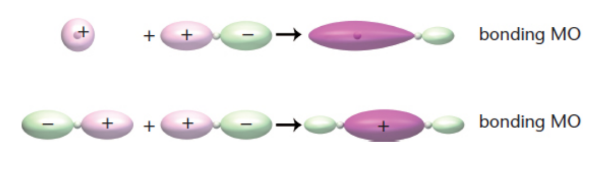
bonding orbital
signs of wave functions are the same; lower energy, more stable; likely to find electrons between atoms

antibonding orbital
signs of wave functions are different; higher energy, less stable; unlikely to find electrons between atoms
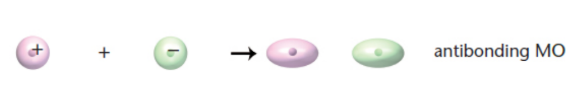
sigma (σ) bond
bonding molecular orbital is formed by head-to-head or tail-to-tail overlap
single bonds
σ bonds, accommodating two electrons; free rotation; longest bond
pi (π) bond
two p-orbitals line up in a parallel (side-by-side) fashion and their electron clouds overlap in a bonding orbital ;cannot exist independently of a σ bond
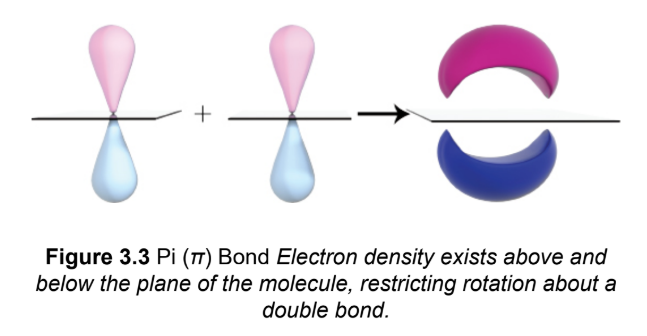
double bond
One π bond on top of an existing σ bond; hinders rotation; medium length bond
triple bond
A σ bond and two π bonds; hinders rotation; shortest bond
orbital hybridization
new orbital shapes formed by mixing different types of orbitals on one atom; a way of making all of the bonds to a central atom equivalent to each other
s-character
the percent of s-orbitals mixing into a hybrid orbital
ex. sp3 has 25%