PMCY 4300 - Exam 3 Study Guide Review (Singh)
1/155
Earn XP
Description and Tags
UGA College of Pharmacy - Uma Singh - Spring 2024; based on professor-provided study guide (Chapters 12-14, 18)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
156 Terms
Define “active principle”
The single chemical in a mixture of compounds chiefly responsible for that mixture’s biological activity
a compound that is isolated from a natural extract and which is principally response for the extract’s pharmacological activity
often used as a lead compound
Define “in vitro testing”
testing procedures carried out on isolated macromolecules, whole cells, or tissue samples
Define “in vivo testing”
studies carried out on animals or humans
Define “high throughput screening”
an automated method of carrying out a large number of in vitro assays on a small scale
a key process used in drug discovery to rapidly test a large number compounds and identify hits from compound libraries, showing potential leads for medicinal chemistry optimization
(when a large number of compounds are tested versus a large number of targets)
Define “prodrug”
A molecule that is inactive in itself, but that is converted to the active drug in the body, normally by an enzymatic reaction
Used to avoid problems related to the pharmacokinetics of the active drug, as well as for targeting
Define “natural product”
a chemical compound coming from nature
Describe the meaning of active principle from a natural product
active principle is a compound that is isolated from a natural extract and is principally responsible for the extracts pharmacological activity
What are the two kinds of target selectivity?
between species
within the body
Describe target selectivity between species
DEF:
identify targets which are unique to the invading pathogen (e.g. penicillin target)
identify targets which are shared, but which are significantly different in structure
EX: antibacterial, antifungal, and antiviral agents
Describe target selectivity within the body
selectivity between different enzymes, receptors, etc.
selectivity between receptor types and subtypes (a-adrenergic receptor, b-adrenergic receptors B1 and B2)
selectivity between isozymes (eNOS vs nNOS)
organ and tissue selectivity (cancer tissue and normal tissue)
What are the benefits of in vitro testing?
more suitable for routine testing
used in high throughput screening
measure the interaction of a drug with the target (but not the ability of the drug to reach the target)
results are easier to rationalize (less factors involved)
What are the limitations of in vitro testing?
does not measure the ability of the drug to REACH the target
does not demonstrate a physiological or clinical effect
does not identify possible side effects
does not identify effective prodrugs
What are the benefits of in vivo testing?
measures an observed physiological effect
measures a drugs ability to interact with its target AND its ability to reach that target
can identify possible side effects
What is a limitation of in vivo testing?
rationalization may be difficult due to the number of factors involved
Which is more expensive, in vitro or in vivo testing?
in vivo
Which is safer, in vitro or in vivo testing?
in vitro
Define “lead compound”
a compound demonstrating a property likely to be therapeutically useful
the level of activity and target selectivity are not crucial
used as the starting point for drug design and development
Define “combinatorial synthesis”
a method that mixes and matches varying building blocks in order to create a vast number of different compounds
done in a sequential process
Define “parallel synthesis”
the simultaneous production of multiple compounds in a single process, enabling rapid exploration of chemical space
Define “de novo drug design”
the process of creating a new drug molecule from scratch, without being based on existing drug structures
What are “me too” drugs?
old drugs are used as starting points to come up with new drug
How do you enhance a side effect?
through selective optimization of side activities (SOSA) of compounds
What does high-throughput screening (HTS) involve?
miniaturization and automation of tests
Define “functional groups”
groups of atoms that contribute to the properties of an organic compound
Define “structure-activity relationships”
The study of the structural features of a drug that are important to its biological activity
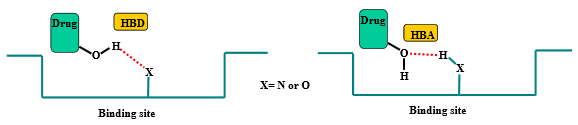
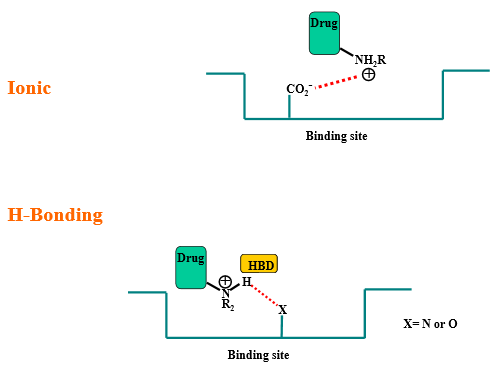
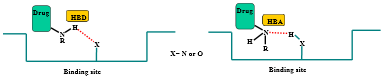
Define “hydrogen bond donor”
A molecule or compound that can donate a hydrogen atom to form a hydrogen bond with another molecule is known as a hydrogen bond donor
hydrogen is typically partially positive as a result of a more electronegative atom (which becomes partially positive)
Define “hydrogen bond acceptor”
A molecule or atom that can form a weak attraction with a hydrogen atom that is already covalently bonded to another electronegative atom
typically partially negative
Define “ionic interaction”
Force of attraction between completely/fully positively and negatively charged ions, resulting in the formation of ionic compounds with high melting and boiling points
Define “van der Waals interactions”
Weak attractive forces between molecules due to temporary dipoles. They include London dispersion, dipole-dipole, and dipole-induced dipole interactions.
Define “pi-pi stacking”
Intermolecular interaction where aromatic rings align face-to-face, creating a stabilizing force due to overlapping π-electron clouds.
Describe the binding interactions of alcohols
Through oxygen atom:
hydrogen bond donor
hydrogen bond acceptor
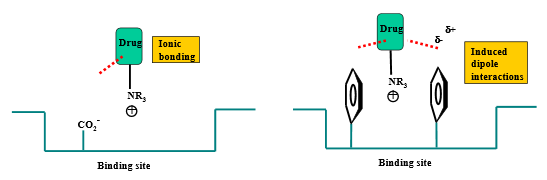
Describe the binding interactions of ionized amines
Through +N-H:
hydrogen bond donor — primary, secondary, and tertiary
ionic bonding — primary, secondary, and tertiary
Describe the binding interactions of unionized amines
Through N-H:
hydrogen bond donor — primary and secondary ONLY
hydrogen bond acceptor — primary, secondary, and tertiary
(T/F) If primary or secondary amines are converted into secondary and tertiary amides, respectively, they will still be able to do ionic bonding (if ionized).
False - amides cannot ionize as their long pair is involved in resonance, so ionic bonding is no longer possible
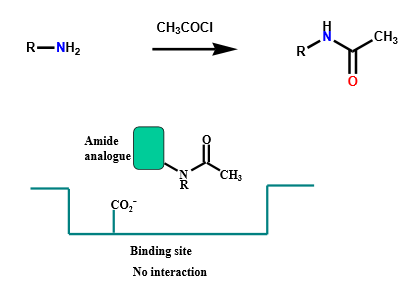
Describe the binding interactions of amides
Through carbonyl oxygen:
hydrogen bond acceptor — primary, secondary, and tertiary
Through N-H:
hydrogen bond donor — primary and secondary ONLY (nitrogen lone pair interacts with neighboring carbonyl group)
acyl group could hinder secondary amide HBD binding

Describe the binding interactions of quaternary ammonium salts
Through +N-H:
ionic bonding
induced dipole interaction (pi stacking)
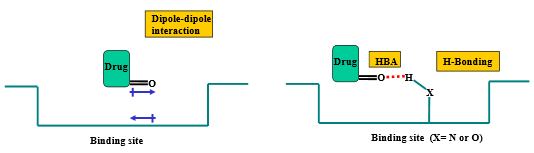
Describe the binding interactions of ketones and aldehydes
Through carbonyl oxygen:
hydrogen bond accepter
dipole-dipole interactions
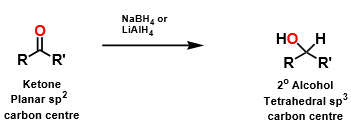
If ketones and aldehydes are reduced to an alcohol, how will that affect the binding interactions of ketones and aldehydes?
will change the geometry from planar to tetrahedral, weakening the interactions (lack of double bond and more hydrogens involved moves oxygen out of range)
however, if still active and can act as a hydrogen bond acceptor, further reactions can be carried out on alcohol to make it an ether or ester
What kind of binding interactions do esters make?
through either oxygen:
hydrogen bond acceptor
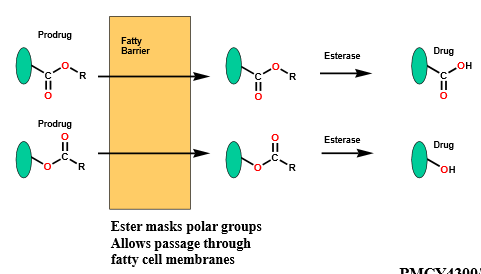
Due to esters’ susceptibility to hydrolysis by esterase, how is this utilized as an advantage in drug design?
can use this to make prodrugs as the molecule’s polarity increases, helping the molecule be more hydrophilic and potentially reach the target easier
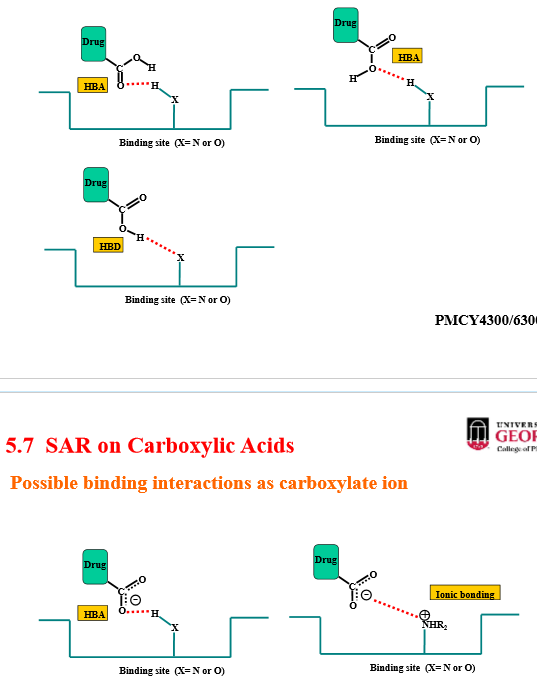
Describe the binding interaction of carboxylic acids
through carbonyl oxygen:
hydrogen bond donor
hydrogen bond acceptor
through hydroxy oxygen (with hydrogen):
weak hydrogen bond acceptor
hydrogen bond acceptor
through ionized hydroxy oxygen (without oxygen):
ionic interaction (anion)
strong hydrogen bond acceptor
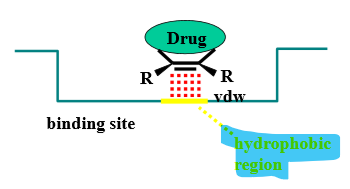
Describe the binding interactions of alkenes
planar & hydrophobic = van der Waals interactions with hydrophobic regions of binding site
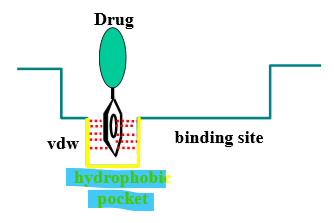
Describe the binding interactions of phenyl/aromatic rings
planar & hydrophobic = van der Waals interactions with FLAT hydrophobic regions of binding site
if the ring is not planar/flat, it is less likely to interact with to hydrophobic regions
Which of the following statements best describes structure-activity relationships (SAR)?
A) The study of which functional groups are important to the chemical reactivity of the drug
B) The study of the physiochemical properties that are important to the absorption of a drug into the blood supply
C) The study of the structural features of a drug that are important to its biological activity
D) The study of the structural features of a drug that are important to its chemical stability
The study of the structural features of a drug that are important to its biological activity
Which of the intermolecular bonding interactions are possible for a secondary amide?
A) hydrogen bonding only
B) van der Waals interactions only
C) ionic bonding only
D) both hydrogen bonding and ionic bonding
hydrogen bonding only
Define “pharmacophore”
the atoms and functional groups required for a specific pharmacological activity, and their relative positions in space
Define “pharmacophore triangles”
Geometric arrangement of three pharmacophoric features in a molecule, crucial for molecular recognition and binding to a target protein.
allows the comparison of molecules that may have the same pharmacophore and binding interactions but use different functional groups to achieve interactions
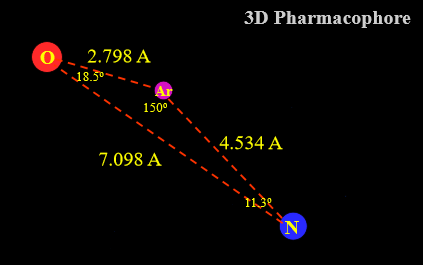
Define “active conformation”
the conformation adopted by a compound when it binds to its target binding site
needed in order to identify the 3D pharmacophore
Why is drug optimization important?
By optimizing binding interactions of the pharmacophore, we can:
increase activity and reduce dose levels
increase selectivity and reduce side effects
What are the different strategies that could be used for optimizing binding interactions?
vary alkyl substituents
vary aryl substituents
extensions (chain or ring)
ring variation
isosteres
simplification
rigidification
Describe how varying alkyl substituents can impact drug design optimization
vary length and bulk of group to either increase binding interactions and selectivity
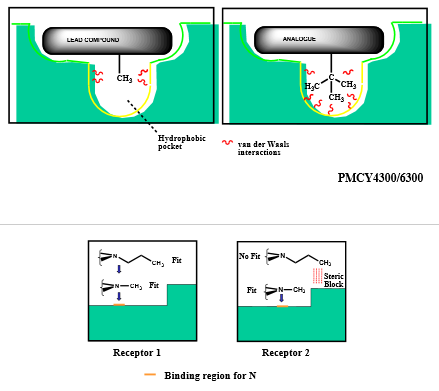
Describe how varying aryl substituents can impact drug design optimization
varying position of substituent on ring can:
increase binding interactions (“reaches” better)
alter electronic effects of induction and resonance (affecting ability to protonate/deprotonate)
Define “inductive effect”
the distribution of electron density along a chain of atoms in a molecule due to differences in electronegativity.
Define “resonance”
where multiple Lewis structures can be drawn for a molecule, showing the delocalization of electrons
What is the difference between the inductive effect and resonance?
Inductive effect: Electron movement through sigma bonds due to electronegativity differences
Resonance: Delocalization of electrons in conjugated systems via pi bonds
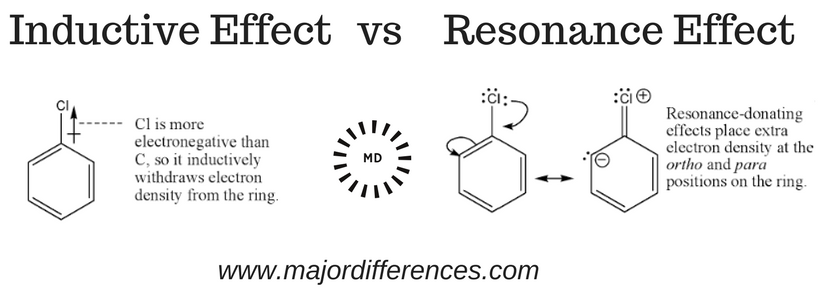
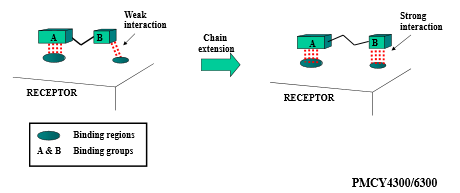
Describe how extension (general) can impact drug design optimization
by adding more functional groups that bind to additional sites, binding interactions are increased (drug can stay attached to site for longer)
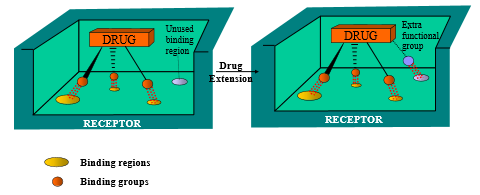
Describe how extension/contraction (chain) can impact drug design optimization
vary length of carbon chain in between two binding groups to either encourage or inhibit binding interactions
Describe how extension/contraction (ring) can impact drug design optimization
vary size of carbon ring (affecting the angles of substituents) to either encourage or inhibit binding interactions and selectivity
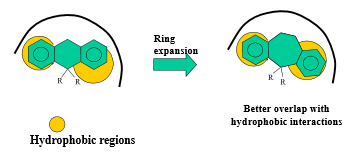
What is another term for extension of a ring?
ring fusion
Describe how ring variations can impact drug design optimization
change the aromatic ring with a heterocyclic ring in order to:
increase binding interactions
increase selectivity
decrease side effects (result of selectivity)
Define “isosteres”
a chemical group considered to be equivalent in physical and chemical properties (especially valency) to another chemical group, maintaining similar enough biological activity
Define “valency”
the number of outer shell (valent) electrons
What are the isosteres of H?
F
D
What are the isosteres of OH?
SH
NH2
CH3
What are the isosteres of O?
S
NH
CH2
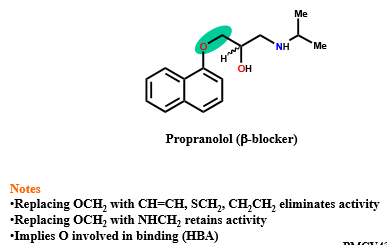
What are the isosteres of CH2CH2?
OCH2
NHCH2
SCH2
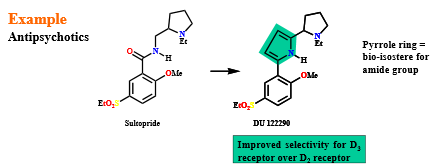
Define “bio-isosteres”
a chemical group that can replace another chemical group without affecting the biological activity of the drug
often used in drug design to replace a problematic group (for a variety of reasons) while retaining activity
What is the difference between isosteres and bio-isosteres?
Isosteres: Compounds with similar shape and charge distribution
Bio-isosteres: (broader term) Molecules with nearly the same biological activity due to similar shape and function
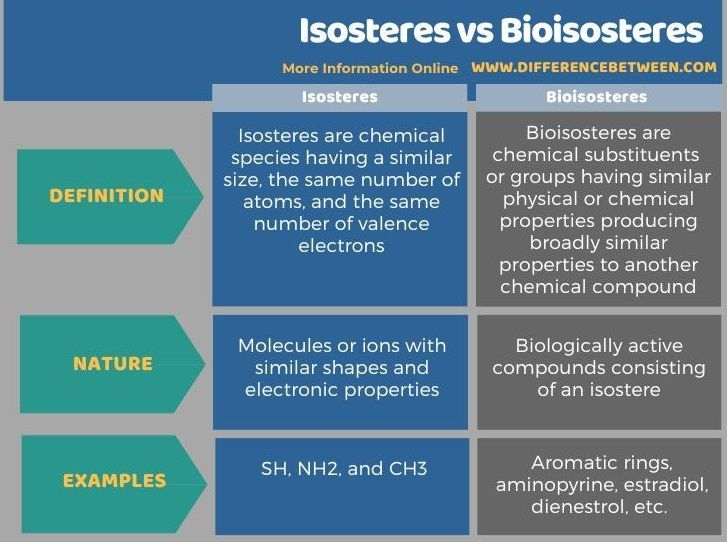
Describe how isosteres can impact drug design optimization
replacing atoms or groups of atoms with physically/chemically-similar ones (and have similar biological activity) can lead to:
more controlled steric properties
more controlled electronic (resonance & induction) properties
altered binding interactions
altered molecular stability
Describe how bio-isosteres can impact drug design optimization
replacing atoms or groups of atoms with physically/chemically-similar ones (and have the same biological activity) can increase:
selectivity
binding interactions
Define “simplification strategies”
the simplification of a drug to remove functional groups, asymmetric centers, and skeletal frameworks that are NOT required for activity
often used on complex lead compounds arising from natural sources
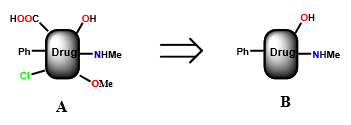
Describe how simplification can impact drug design optimization
removing functional groups, asymmetric centers, and or shortening/simplifying the carbon framework can:
streamline synthesis/manufacturing of compound
increase binding interactions (activity)
increase selectivity (less toxicity)
(T/F) Simplification occurs all at a single time.
False - done in stages in order to avoid oversimplification (need to test at each stage to make sure biological activity is still retained)
What are the disadvantages of simplification strategies?
oversimplification may result in decreased activity and selectivity
simpler molecules have more conformations (more flexible)
more likely to interact with more than one target binding site (lead compound has been generalized a little too much)
may result in increased side effects (due to decreased selectivity)
Define “rigidification strategies”
used to limit the number of conformations that a drug can adopt while retaining the active conformation
achieved by introducing a ring structure, rigid functional groups, or steric blockers
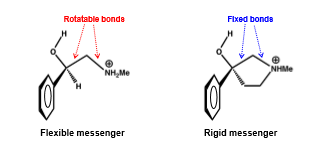
How is rigidification achieved?
introducing a ring structure
introducing rigid functional groups
steric blockers
Give examples of rigidification via rigid functional groups
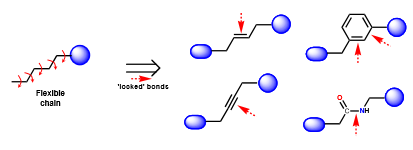
Define “steric/conformational blockers” in relation to rigidification
groups that are added to molecules to prevent them adopting certain conformations by introducing steric clashes (electrons repel each other)
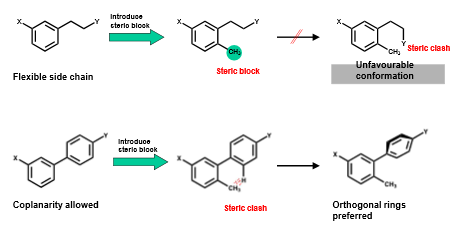
What is the risk with using steric/conformational blockers in rigidification?
steric blocking may disallow an active conformation instead of favoring it
There are three general methods by which molecules can be simplified. Which of the following IS one of the methods?
A) addition of excess rings
B) removal of excess flexibility
C) addition of excess functional groups
D) removal of asymmetric centers
removal of asymmetric centers - A & are rigidification and C is extension (general)
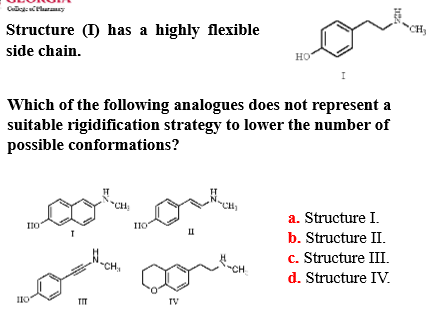
Structure 1 (see photo) has a highly flexible side chain.
Which of the following analogues does NOT represent a suitable rigidification strategy to LOWER the number of possible conformations?
A) Structure I
B) Structure II
C) Structure III
D) Structure IV
Structure IV - added a heterocyclic ring on the side of the structure that did not require rigidification, and could potentially impact binding interactions/biological activity/selectivity
Describe how rigidification can impact drug design optimization
decreasing the ability to rotate bonds by locking them in rings, adding rigid functional groups, or adding steric blockers can increase:
activity (more chance of desired active conformation being present)
selectivity (less chance of undesired active conformation, leading to less side effects)
What is the disadvantage of rigidification?
molecule is more complex and may be more difficult to synthesize
Why are esters replaced with amides or with groups that cause steric hindrance?
to improve metabolic stability of molecules and its biological activity, as esters are hydrolyzed in the body really quickly
Define “steric hindrance”
the repulsion between electron clouds in atoms that are brought too close together, leading to a decrease in reaction rate or yield
What is the difference between pharmacokinetics and pharmacodynamics?
Pharmacokinetics: focuses on drug absorption, distribution, metabolism, and excretion in the body (body on drug)
Pharmacodynamics: studies the drug's effects on the body and its mechanism of action (drug on body)
Define “pharmaceutical phase”
the disintegration of a tablet or capsule in the GI tract, the release of the drug, and its dissolution
Define “pharmacokinetic phase”
absorption of a drug from the GI tract into the blood supply, the various factors that affect the drug’s survival and progress as it travels to its molecular target
Define “pharmacodynamic phase”
the mechanism of drug interaction with its molecular target and the resulting pharmacological effect"
How can you improve the pharmacokinetic properties of a lead compound?
optimize chemical and metabolic stability
optimize hydrophilic/hydrophobic balance
drug needs to be sufficiently polar to be soluble in aqueous conditions
drug needs to be sufficiently nonpolar/fatty to cross cell membranes and avoid rapid excretion
optimize solubility
optimize drug half-life
optimize distribution characteristics
How can you alter the hydrophobicity and hydrophilicity of drugs?
by changing the functional groups
vary alkyl substituents
“masking” or removing polar groups
adding polar groups
vary pKa
Describe how varying alkyl substituents can impact solubility and membrane permeability (hydrophilic/hydrophobic properties)
polarity can be affected (increased or decreased) by the addition, removal, or variation of suitable hydrophobic (alkyl) substituents
What is a common example of varying alkyl substituents for impacting hydrophilic/hydrophobic properties and how does it work?
methylene shuffle -one alkyl chain is shortened by one carbon unit, while another is lengthened by one carbon unit
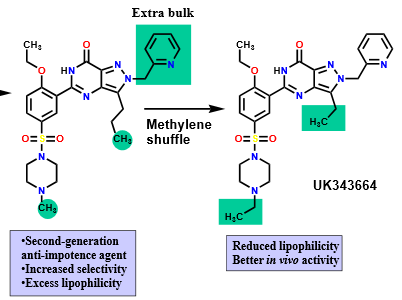
Describe how “masking” or removing polar groups can impact solubility and membrane permeability (hydrophilic/hydrophobic properties)
by masking a polar functional group with an alkyl or acyl group:
decrease polarity
increase hydrophobic character
What are the disadvantages of “masking” or removing polar groups?
polar group may be involved in target binding (decreasing binding interactions and activity)
unnecessary polar groups are likely to have been removed already due to the simplification strategy
Describe how adding polar groups can impact solubility and membrane permeability (hydrophilic/hydrophobic properties)
by adding a polar group:
increase polarity
decrease hydrophobic character
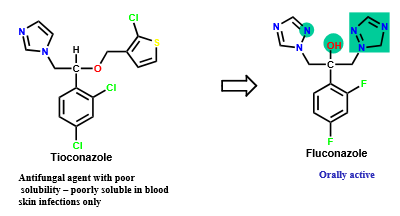
What kind of situations are best for adding a polar group to a drug in order to optimize its hydrophilic/hydrophobic properties?
targeting drugs against gut infections
useful for reducing CNS side effects
What is a disadvantage of adding polar groups?
may introduce unwanted side effects
Describe how varying pKa can impact solubility and membrane permeability (hydrophilic/hydrophobic properties)
varying pKA alters percentage of drug which is ionized, which can impact solubility
if aromatic amine or carboxylic acid
add electron-donating or electron-withdrawing substituents to ring
position is important if substituent interacts with the ring through resonance
vary aryl substituents
if non-aromatic system (like amine nitrogens)
add electron-donating or electron-withdrawing groups
vary alkyl substituents
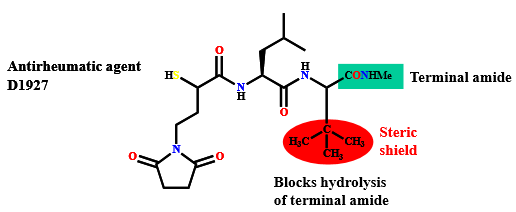
Describe how steric shields can impact drug stability (degradation resistance)
by introducing a bulky group:
increase chemical and metabolic stability
protect a susceptible functional group from hydrolysis by hindering attack by nucleophiles or enzymes