3.2.2 reaction rates
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
collision theory
for a reaction to occur successfully the articles must collide in right direction and have energy greater than or equal to activation energy
rate of reaction
how fast a reaction takes lace
rate of reaction equation
amount of reactant used or product formed over time
how to work out rate of reaction from grah
gradient of curve at the given point
draw a tangent if curved then calculate gradient
how does temperature affect rate of reaction
when a substance is heated thermal energy is transferred this energy is converted to kinetic energy so molecules move faster and further so more collisions with greater energy so more collisions have energy greater than that activation energy so increase rate as more succesful
how does concentration and ressure increase reaction rate
concentration increases more molecules in same volume so acked closer so collisions more likely and robability of collision occuring with energy greater than Ea more likely so rate of reaction increases
increase ressure ha same effect as packed closer together in smaller vlume
effect of surface area on rates of reaction
increasing surface area increases number of exposed particles so more frequenet and succesful collisions
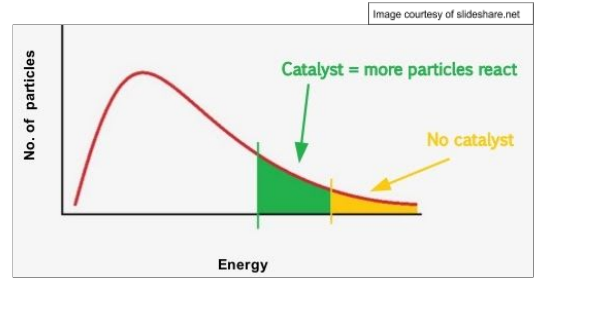
catalyst
substance that increases rate of reaction without being used up by providing and alternative reaction ath with lower activation energy
benefits of catalyst
lower energy costs as alow lower temperature and pressure to be use
give higher atom economy
increase sustainability as reduce energy demand from combustion of fossil fuels so lower CO2 emissions
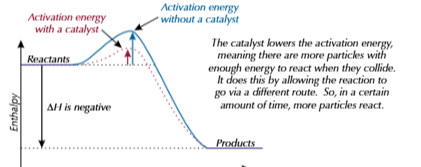
reaction rofile for catalyst
homogeneous catalyst
same hysical state as reactant
heterogeneous catalysts
in a different hase than reactants eg hysical state
why are transition metals good catalysts
varable oxidation states
electrons are transferred to roduce and reactive intermediatie to seed u reaction rate
how does a catalyst work
adsorbing molecules onto an active site on the surface of the catalyst these active sites increas the roximity of molecules and weaken covalent bonds in the molecules so reaction occur more easily and rate is increased
ways to investigate reaction rates
when roduct is gas formation measured using mass valance,start timer and measure at regular time intervals and when reading on mass stos decreasing sto timer
if gas given off collect in gas syringe and calculate jow much you have at regular time intervals
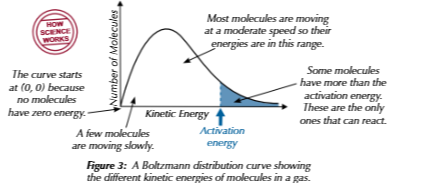
maxwellboltzmann distirubtion
not all molecules in substance have same amunt of energy so distributed in attern called this ,numvber of moleculea against kinetic energy
boltzmann distribution grah
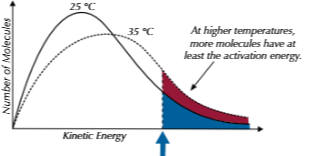
effect of temerature on boltzmann distribution
shifts right as greater roortion of molecules have greater kinetic energy
effect of concentration,ressure and surface area on boltzmann distribution
nothing as doesnt change energy
effect of catalyst on boltzamann
activation energy shifted left