IB Chem: Topic 1.1-1.3 Practice Questions PART 1
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms
C. Ethanol and water
Which is a homogeneous mixture?
A. Oil and water
B. Sand and water
C. Ethanol and water
D. Chalk and sand
C. C₁₀H₂₂
What is the molecular formula of a hydrocarbon containing 84.6% carbon by mass with a molar mass of 142.3 g mol⁻¹?
A. C₂₀H₄₄
B. C₁₁H₁₀
C. C₁₀H₂₂
D. C₅H₁₁
A
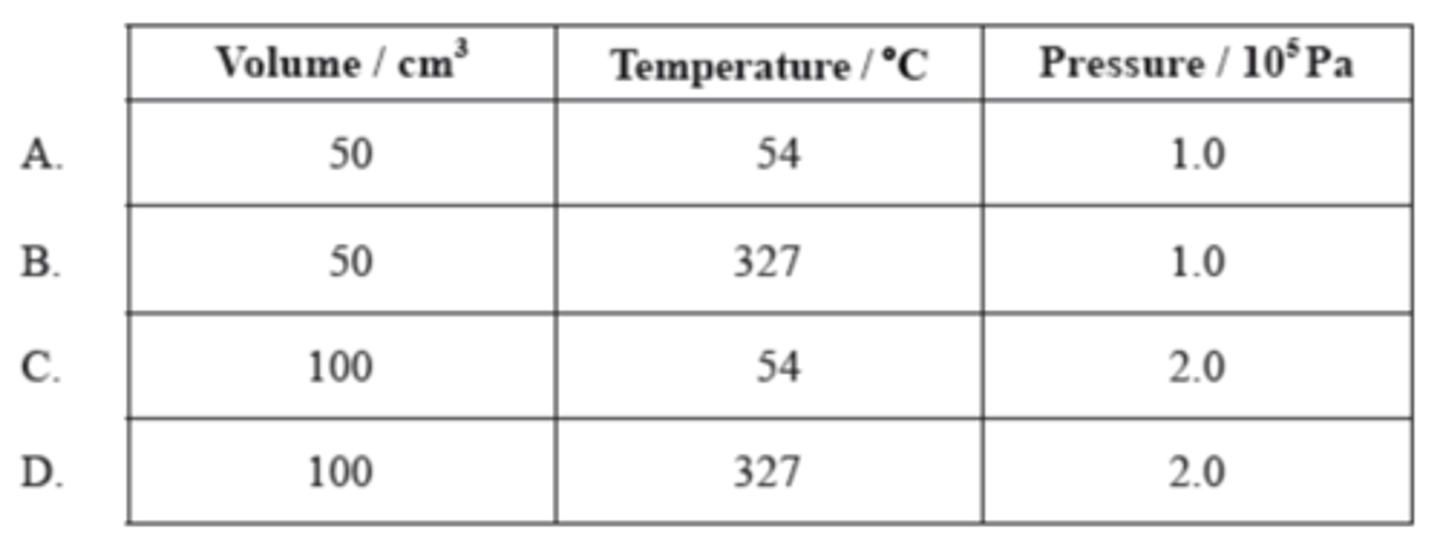
Which graph shows the relationship between the volume and pressure of a fixed mass of an ideal gas?
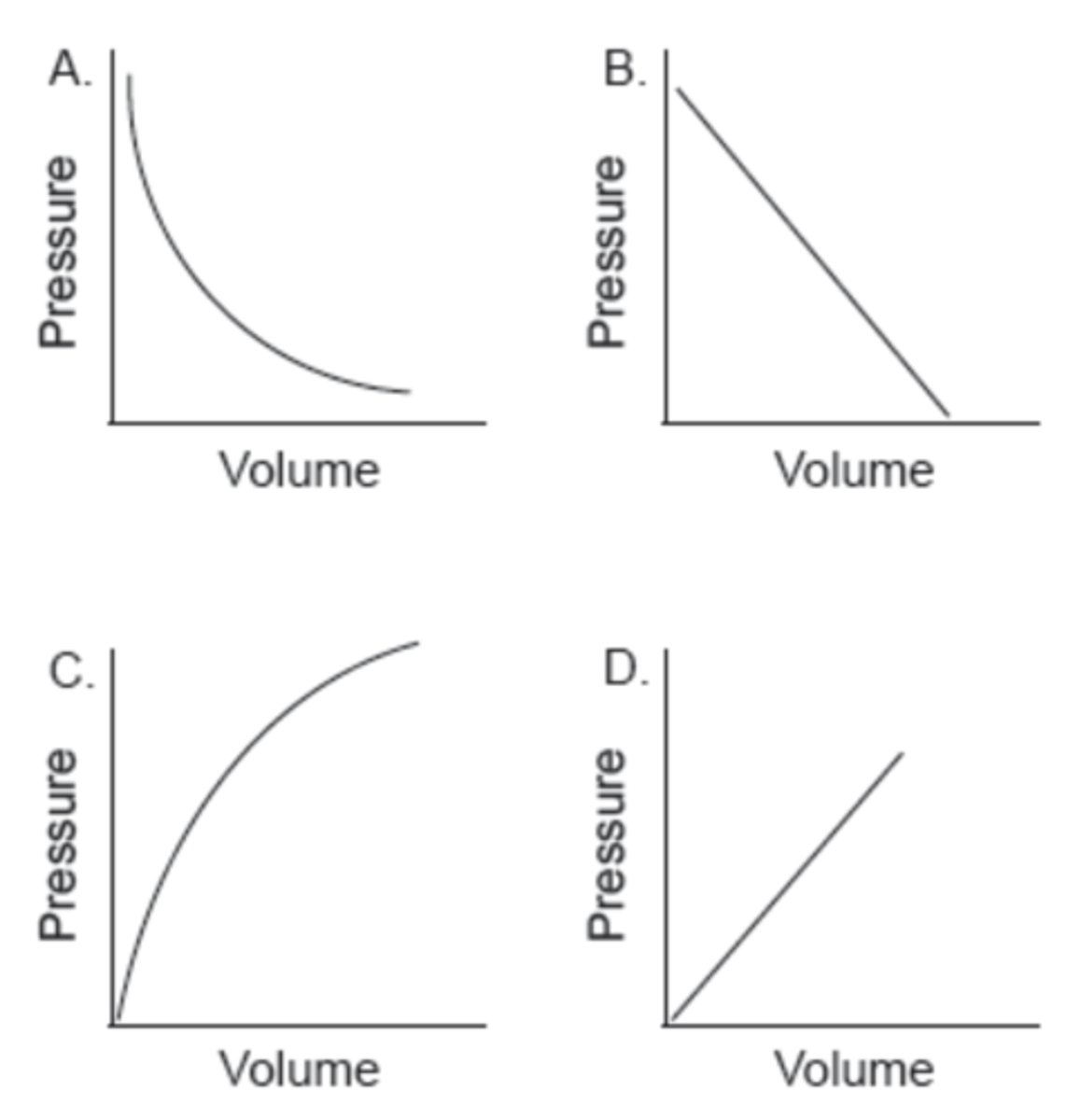
D
What is the percentage yield when 7 g of ethene produces 6 g of ethanol?
Mr(ethene) = 28 and Mr(ethanol) = 46
C₂H₄(g) + H₂O(g) → C₂H₅OH(g)

C. 8
What is the sum of the coefficients when the equation is balanced with the lowest whole number ratio?
__Na₂S₂O₃(aq) + __HCl(aq) → __S(s) + __SO₂(g) + __NaCl(aq) + __H₂O(l)
A. 6
B. 7
C. 8
D. 9
D. 1.6 × 10²⁵
What is the number of atoms of oxygen in 2.0 mol of hydrated sodium carbonate, Na₂CO₃•10H₂O
Avogadro's constant, L or NA: 6.02 × 10²³ mol-1
A. 6
B. 26
C. 3.6 × 10²⁴
D. 1.6 × 10²⁵
C. 500
What is the volume, in cm³, of the final solution if 100 cm³ of a solution containing 1.42 g of sodium sulfate, Na₂SO₄, is diluted to the concentration of 0.020 mol dm⁻³?
Mr(Na₂SO₄) = 142
A. 50
B. 400
C. 500
D. 600
B
What is the percentage yield when 2.0 g of ethene, C₂H₄, is formed from 5.0 g of ethanol, C₂H₅OH?
Mr(ethene) = 28; Mr(ethanol) = 46
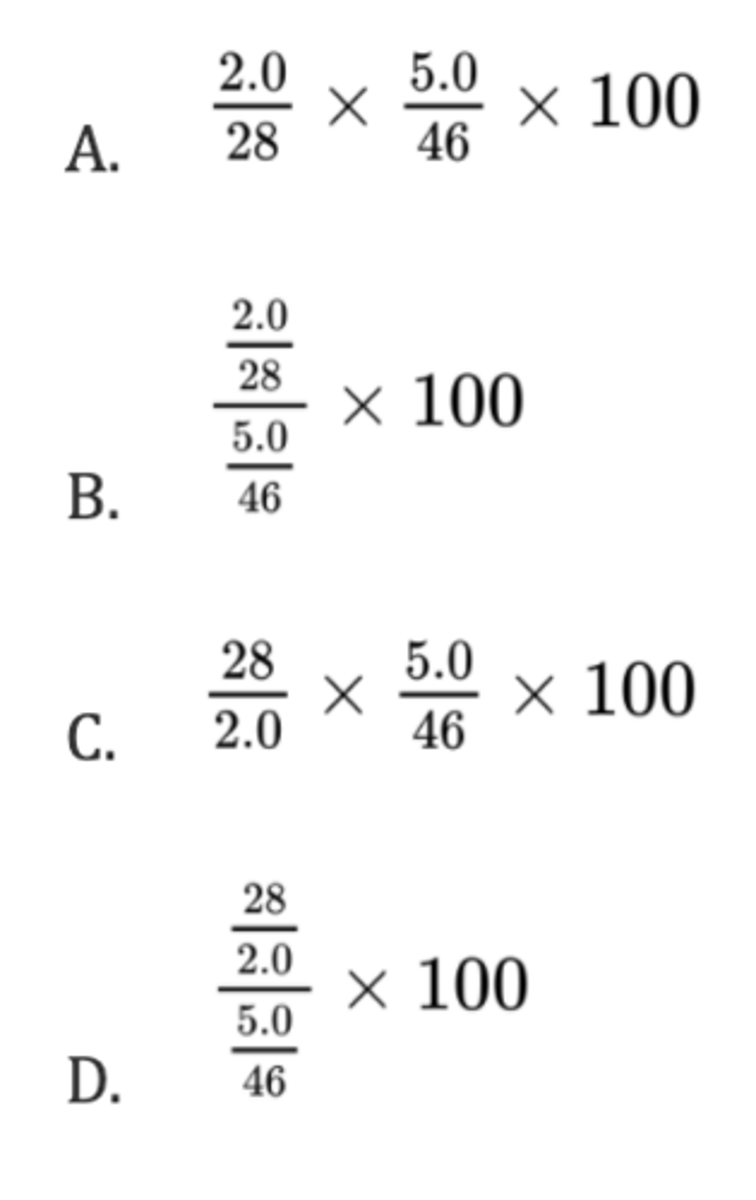
A
Which electron transition emits energy of the longest wavelength?
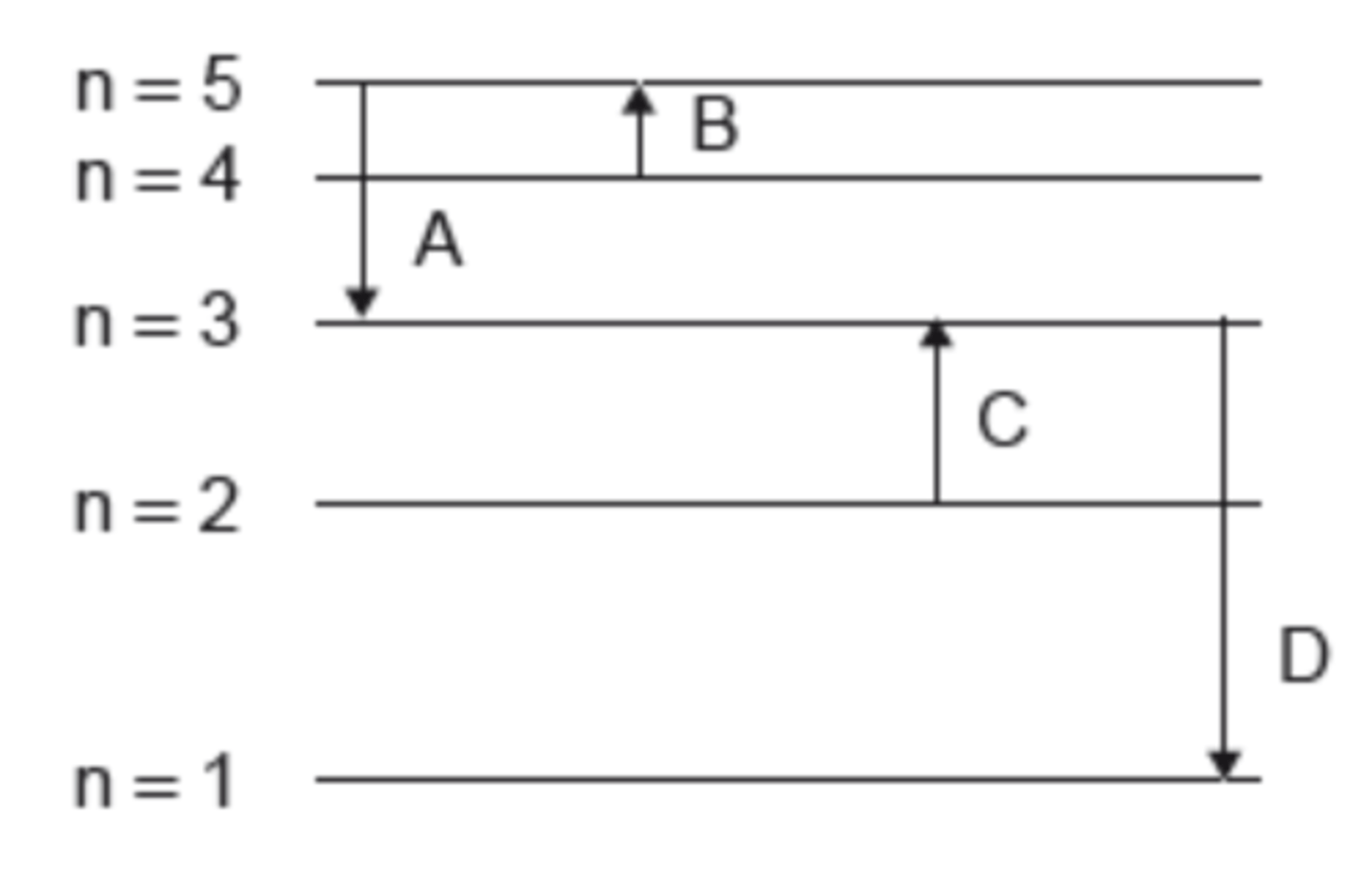
D. 6.02 × 10²³
How many atoms of nitrogen are there in 0.50 mol of (NH₄)₂CO₃?
A. 1
B. 2
C. 3.01 × 10²³
D. 6.02 × 10²³
D. 10
What is the value of x when 32.2 g of Na₂SO₄ • xH₂O are heated leaving 14.2 g of anhydrous Na₂SO₄?
Mr(H₂O) = 18; Mr(Na₂SO₄) = 142.
Na₂SO₄ • xH₂O (s) → Na₂SO₄ (s) + xH₂O (g)
A. 0.1
B. 1
C. 5
D. 10
C. 130.0
How many grams of sodium azide, NaN₃, are needed to produce 68.1 dm³ of N₂ (g) at STP?
Molar volume at STP = 22.7 dm³ mol⁻¹; Mr(NaN₃) = 65.0
2NaN₃ (s) → 3N₂ (g) + 2Na (s)
A. 32.5
B. 65.0
C. 130.0
D. 195.0
B. 5
What is the sum of the coefficients when the following equation is balanced using the smallest whole numbers?
__C₆H₁₂O₆ (aq) → __C₂H₅OH (aq) + __CO₂ (g)
A. 4
B. 5
C. 9
D. 10
A. N₂H₄
Which compound has the greatest percentage by mass of nitrogen atoms?
A. N₂H₄
B. NH₃
C. N₂O₄
D. NaNO₃
B. I and III only
Which statements about mixtures are correct?
I. The componenets may be elements or compounds.
II. All components must be in the same phase.
III. The components retain their individual properties.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
C. 0.020
5.0 cm³ of 2.00 mol dm⁻³ sodium carbonate solution, Na₂CO₃(aq), was added to a volumetric flask and the volume was made up to 500 cm³ with water. What is the concentration, in mol dm⁻³, of the solution?
A. 0.0050
B. 0.0040
C. 0.020
D. 0.010
B
What is the expression for the volume of hydrogen gas, in dm³, produced at STP when 0.30 g of magnesium reacts with excess hydrochloric acid solution?
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Molar volume of an ideal gas at STP = 22.7 dm³mol⁻¹

C. 53
What is the sum of the coefficients when the equation is balanced with whole numbers?
__C₈H₁₈(g) + __O₂(g) → __CO(g) + __H₂O(l)
A. 26.5
B. 30
C. 53
D. 61
B. 7.00
How many moles of oxygen atoms are there in 0.500 mol of hydrated iron(II) ammonium sulfate, (NH₄)₂Fe(SO₄)₂•6H₂O(s)?
A. 4.00
B. 7.00
C. 8.00
D. 14.00
C
What is the maximum volume, in dm³, of CO₂(g) produced when 1.00 g of CaCO₃(s) reacts with 20.0 cm³ of 2.00 mol dm⁻³ HCl(aq)?
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g)
Molar volume of gas = 22.7 dm³mol⁻¹; Mr(CaCO₃) = 100.00
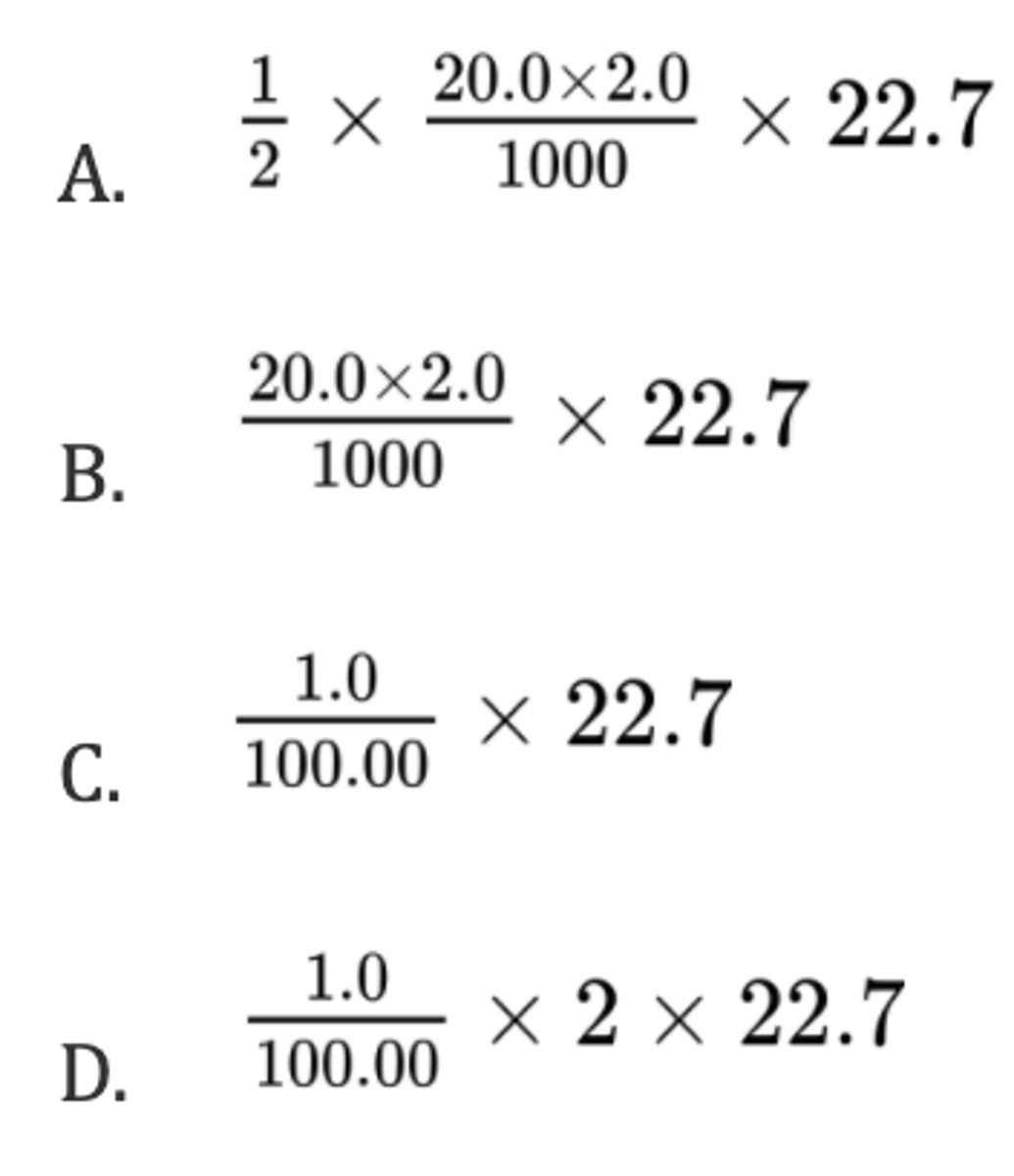
A. I and II only
Which factors affect the molar volume of an ideal gas?
I. Pressure
II. Temperature
III. Empirical formula
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
C. 25.0 cm³ of 0.120 mol dm⁻³ H₂SO₄
Which solution neutralizes 50.0 cm³ of 0.120 mol dm⁻³ NaOH (aq)?
A. 12.5 cm³ of 0.080 mol dm⁻³ H₃PO₄
B. 25.0 cm³ of 0.120 mol dm⁻³ CH₃COOH
C. 25.0 cm³ of 0.120 mol dm⁻³ H₂SO₄
D. 50.0 cm³ of 0.060 mol dm⁻³ HNO₃
D
What is the pressure, in Pa, inside a 1.0 m³ cylinder containing 10 kg of H₂ (g) at 25 ºC?
R = 8.31 J K⁻¹ mol⁻¹; pV = nRT
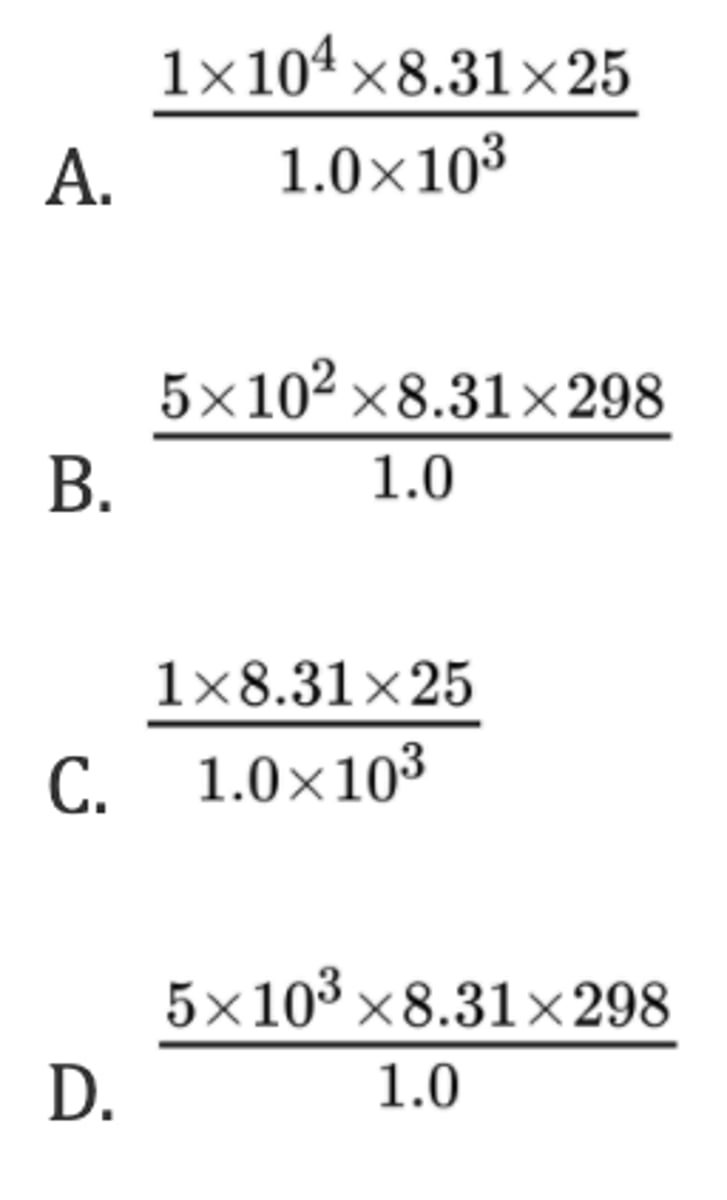
C. C₅H₁₀O₂
A compound with Mr = 102 contains 58.8 % carbon, 9.80 % hydrogen and 31 % oxygen by mass. What is its molecular formula?
Ar: C = 12.0; H = 1.0; O = 16.0
A. C₂H₁₄O₄
B. C₃H₄O₄
C. C₅H₁₀O₂
D. C₆H₁₄O
B. HgCl₂(s) → HgCl₂(g)
Which equation represents sublimation?
A. 2Al(s) + 3I₂(g) → 2AlI₃(s)
B. HgCl₂(s) → HgCl₂(g)
C. I₂(g) → I₂(s)
D. CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + CO₂(g)+ H₂O(l)
D. 500
Which volume, in cm³, of 0.20 mol dm⁻³ NaOH (aq) is needed to neutralize 0.050 mol of H₂S(g)?
H₂S(g) + 2NaOH(aq) → Na₂S(aq) + 2H₂O(l)
A. 0.25
B. 0.50
C. 250
D. 500
D
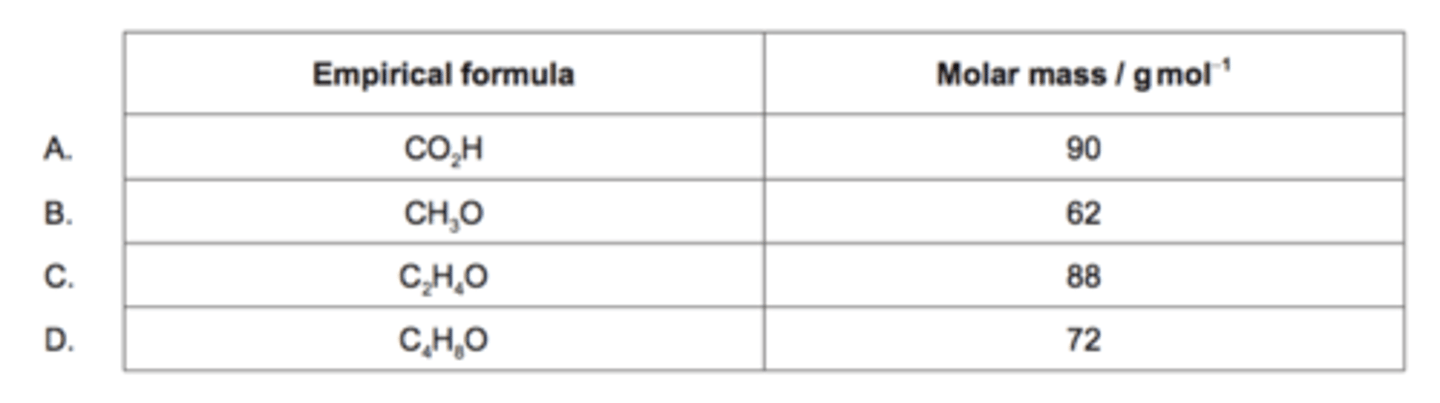
For which compound is the empirical formula the same as the molecular formula?
Ar(H)=1; Ar(C)=12; Ar(O)=16
C. C₄H₁₀
The complete combustion of 15.0 cm³ of a gaseous hydrocarbon X produces 60.0 cm³ of carbon dioxide gas and 75.0 cm³ of water vapour. What is the molecular formula of X? (All volumes are measured at the same temperature and pressure.)
A. C₄H₆
B. C₄H₈
C. C₄H₁₀
D. C₆H₁₀
B. 0.10mol NaOH + 0.10mol H₂SO₄
In which mixture is NaOH the limiting reagent?
A. 0.20mol NaOH + 0.10mol H₂SO₄
B. 0.10mol NaOH + 0.10mol H₂SO₄
C. 0.20mol NaOH + 0.10mol HNO₃
D. 0.10mol NaOH + 0.10mol HNO₃
A. Molecules have finite volume.
Why do gases deviate from the ideal gas law at high pressures?
A. Molecules have finite volume.
B. Cohesive forces increase the volume from the ideal.
C. Increasing pressure increases the temperature of the gas.
D. Collisions between molecules occur more frequently as pressure increases.
B
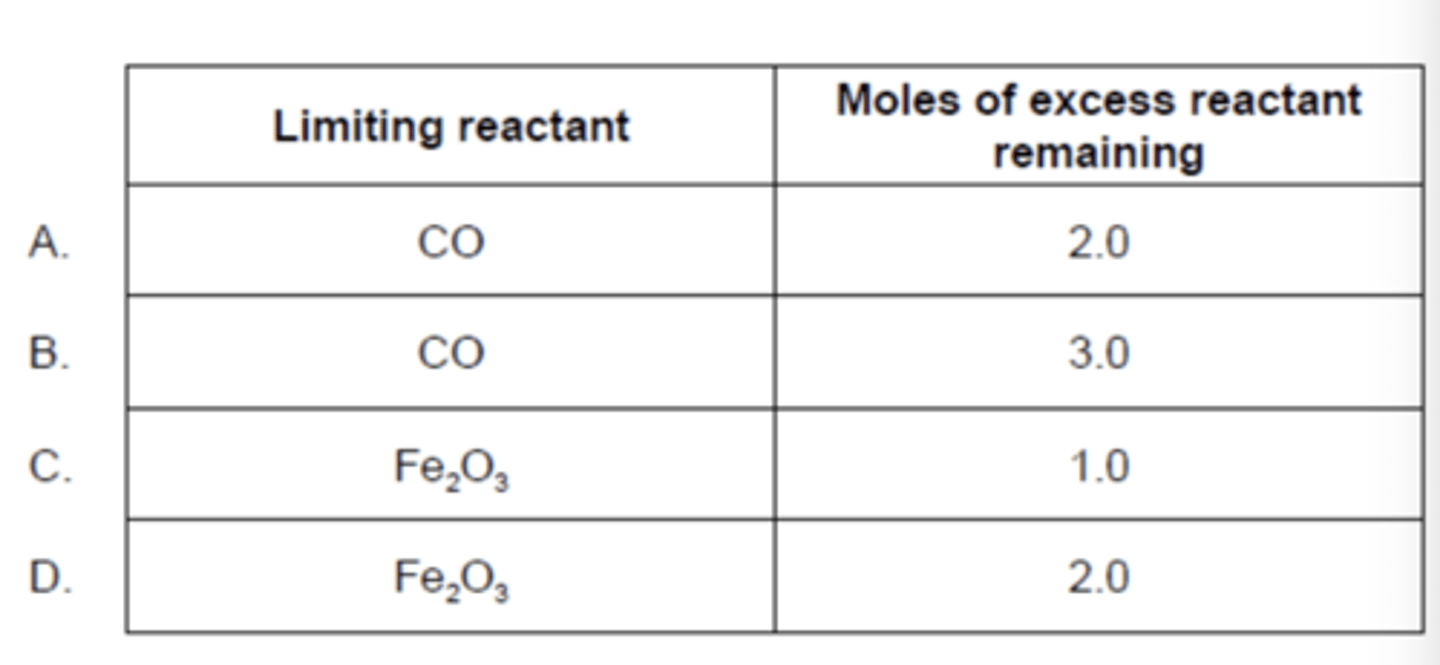
5.0mol of Fe₂O₃(s) and 6.0mol of CO(g) react according to the equation below. What is the limiting reactant and how many moles of the excess reactant remain unreacted?
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
C. 1:3
Combustion of ethanol takes place according to the following unbalanced equation.
__C₂H₅OH(l) + __O₂(g) → __CO₂(g) + __H₂O(l)
What is the mole ratio of ethanol to oxygen in the balanced equation?
A. 1:1
B. 2:1
C. 1:3
D. 2:7
A. 0.20 mol P₂O₅
Which sample contains the largest amount, in mol, of oxygen atoms?
A. 0.20 mol P₂O₅
B. 0.30 mol O₃
C. 0.40 mol CH₃COOH
D. 0.80 mol H₂O
D. C₆H₆
Which compound has the highest percentage of carbon by mass?
A. CH₄
B. C₂H₄
C. C₄H₁₀
D. C₆H₆
B
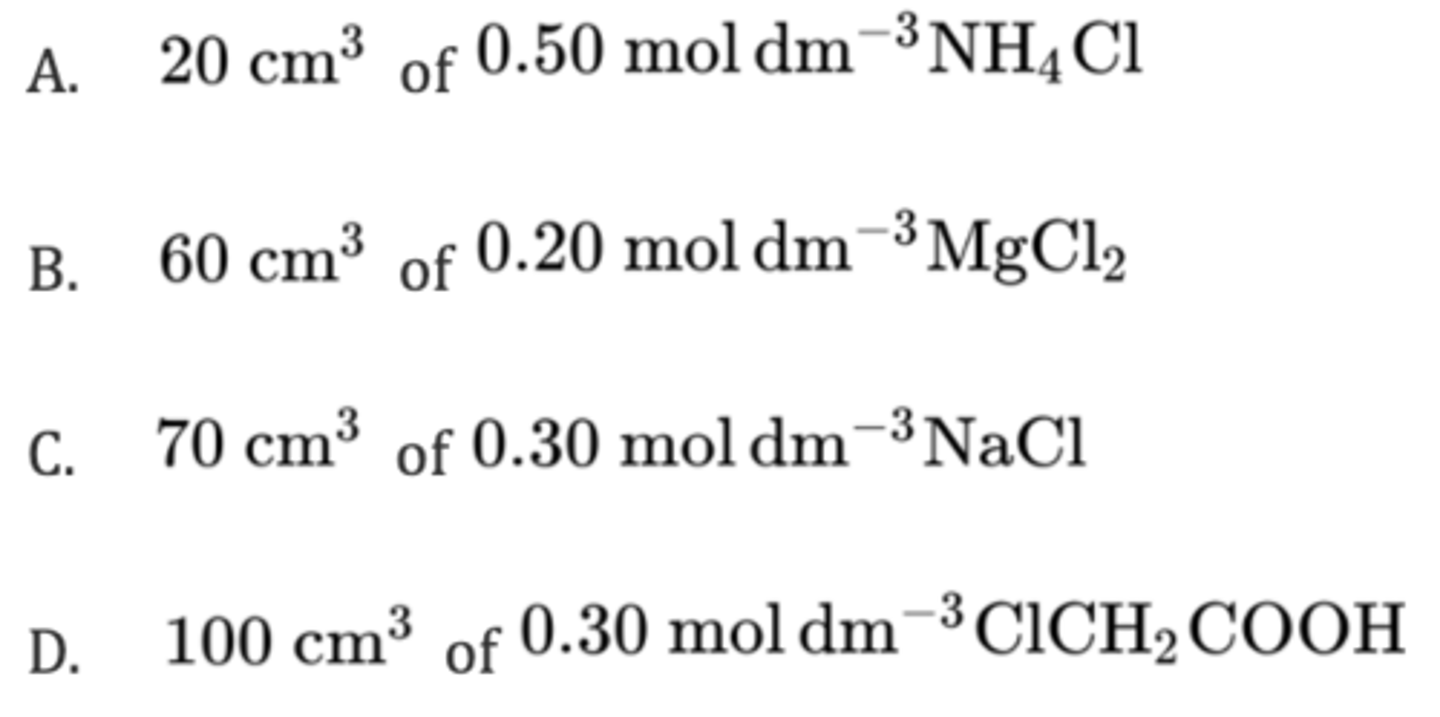
Which solution contains the biggest amount, in mol, of chloride ions?
D. 2.4 x 10²⁴
What is the total number of protons and electrons in one mole of hydrogen gas?
A. 2
B. 4
C. 1.2 x 10²⁴
D. 2.4 x 10²⁴
B. CH₂
A hydrocarbon contains 85.7 % carbon by mass. What is the empirical formula of the hydrocarbon?
A. C₂H₃
B. CH₂
C. C₂H₅
D. CH₃
C. 13
What is the sum of all coefficients for the combustion of one mole of propane?
__C₃H₈(g) + __O₂(g) → __CO₂(g) + __H₂O(l)
A. 8
B. 12
C. 13
D. 15
A
A gas with a molar mass (M) of 44 g mol⁻¹ occupies a volume of 2.00 x 10³ cm³ at a pressure of 1.01 x 10⁵ and a temperature of 25 °C. Which expression is correct for the calculation of the mass of the gas, in g?
R = 8.31 J K⁻¹ mol⁻¹

C. Excess remains in solution.
4.0 g of solid sodium hydroxide is added to 0.10 dm³ of 1.0 mol dm⁻³ aqueous sulfuric acid.
2NaOH(s) + H₂SO₄(aq) → Na₂SO₄(aq) + 2H₂O(l)
Which statement is correct?
A. Neither reactant is in excess.
B. 0.10 mol is formed.
C. Excess remains in solution.
D. Excess NaOH remains in solution.
C
Which expression gives the sum of all the coefficients for the general equation for the complete
combustion of hydrocarbons?
__CxHy(g) + __O₂ → __CO₂(g) + __H₂O(l)
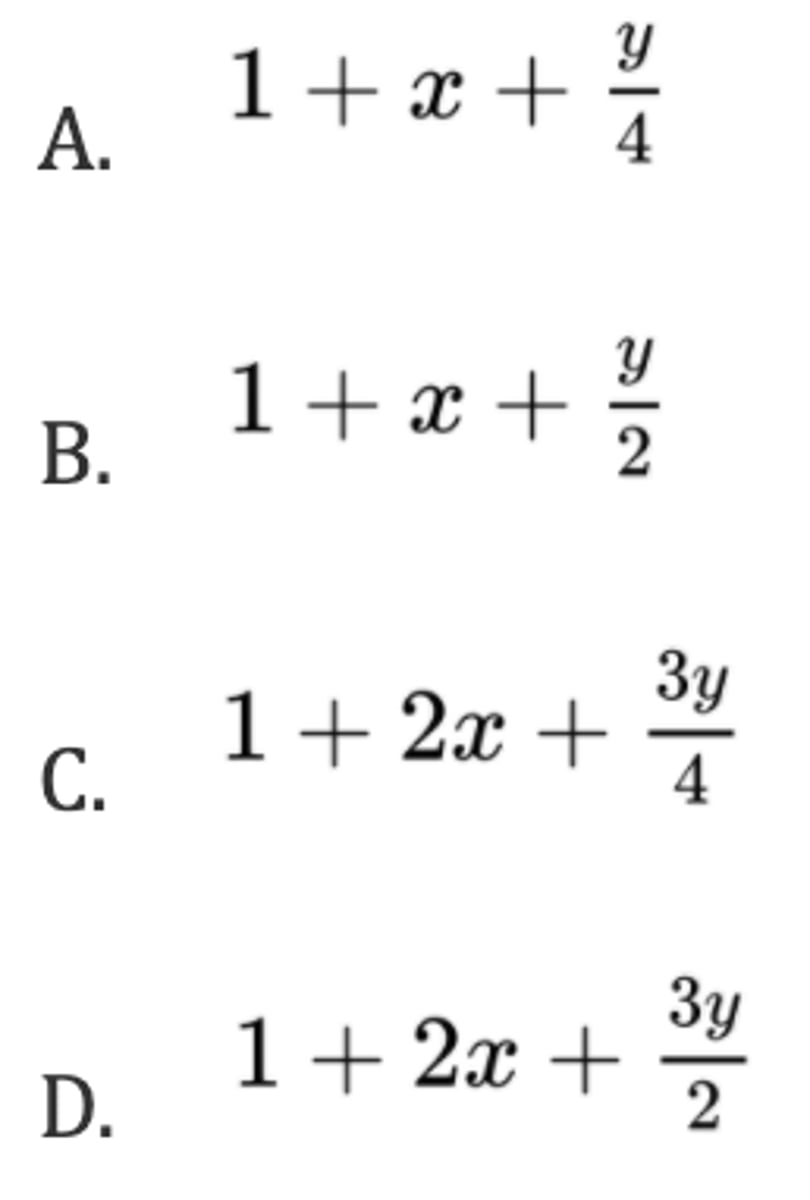
D. 0.40
0.040 mol of (NH₄)₂Ni (SO₄)₂ • 6H₂O is dissolved in water to give 200 cm³ of aqueous solution. What is the concentration, in mol dm⁻³, of ammonium ions?
A. 0.00040
B. 0.0080
C. 0.20
D. 0.40
B. 0.067
When sodium bromate(V), NaBrO₃, is heated, it reacts according to the equation below.
2NaBrO₃(s) → 2NaBr(s) + 3O₂(g)
What amount, in mol, of NaBrO₃ produces 2.4 dm³ of oxygen gas, measured at room temperature and pressure? (Molar volume of gas = 24 dm³mol⁻¹ at room temperature and pressure.)
A. 0.017
B. 0.067
C. 0.10
D. 0.15
D. 20
Aluminium carbide reacts with water according to the equation below. What is the sum of all the coefficients when the equation is balanced?
__Al₄C₃(s) + __H₂O(l) → __Al(OH)₃(s) + __CH₄(g)
A. 13
B. 14
C. 19
D. 20
D. 800 K
At which temperature, in K, assuming constant pressure, is the volume of a fixed mass of gas at 127 °C doubled?
A. 200 K
B. 254 K
C. 400 K
D. 800 K
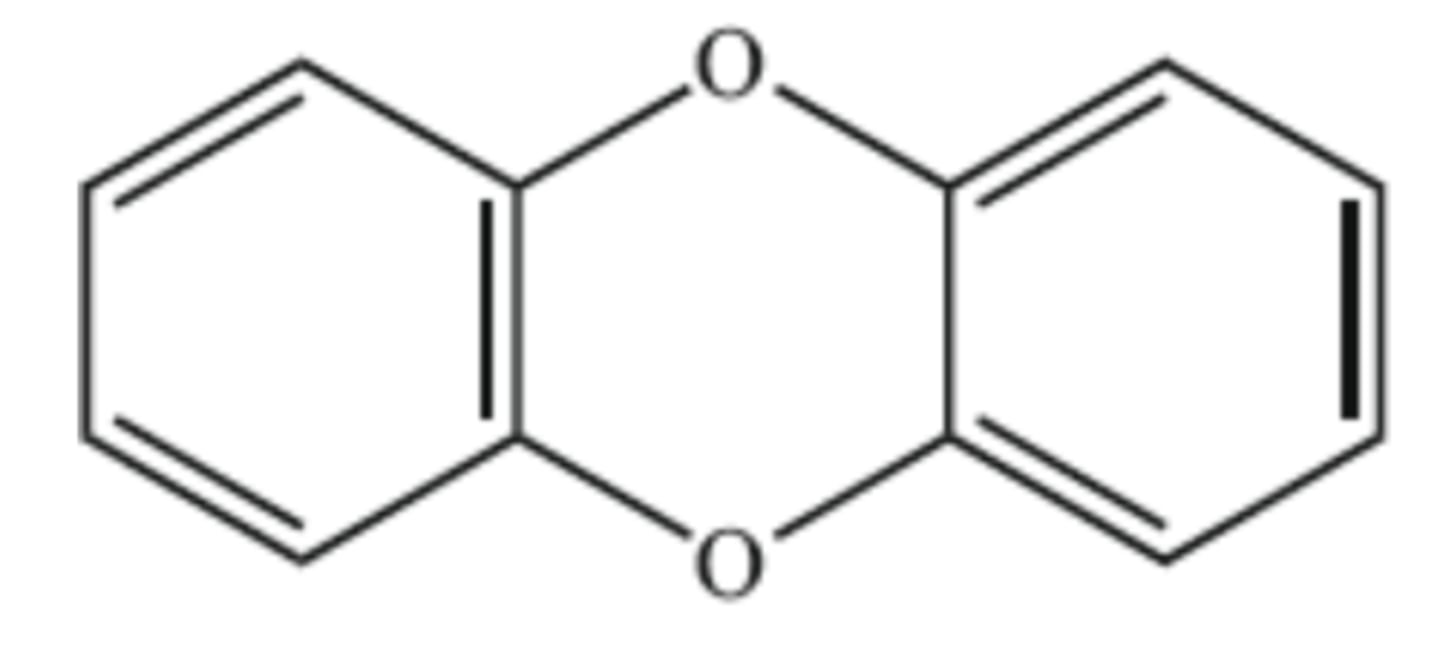
B. C₆H₄O
The structural formula of a dioxin is shown below. What is its empirical formula?
A. C₆O
B. C₆H₄O
C. C₆H₆O
D. C₁₂H₈O₂
A. 0.0050
100.0 cm³ of a 0.50 mol dm⁻³ solution of BaCl₂ is added to 50.0 cm³ of a 0.10 mol dm⁻³ solution of Na₂SO₄. A precipitate of BaSO₄ is formed according to the equation below.
BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq)
What is the amount, in mol, of BaSO₄ produced?
A. 0.0050
B. 0.010
C. 0.050
D. 0.10
B. I and III only
Which volumes of gases at standard temperature and pressure have the same mass as 100 cm³ of O₂?
I. 50 cm³ of SO₂
II. 100 cm³ of CH₄
III. 100 cm³ of SiH₄
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
D
What is the mass, in g, of one mole of hydrated copper(II) sulfate, CuSO₄•5H₂O, given the following relative atomic mass values?
A. 160
B. 178
C. 186
D. 250
B. I and III only
For which compounds is the empirical formula the same as the molecular formula?
I. Methane
II. Ethene
III. Ethanol
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
A. Solute
Some sodium chloride is dissolved in water. Which term describes the role of sodium chloride in this process?
A. Solute
B. Solvent
C. Solution
D. Saturated
D. 800 K
At which temperature, in K, assuming constant pressure, is the volume of a fixed mass of gas at 127 °C doubled?
A. 200 K
B. 254 K
C. 400 K
D. 800 K
D
Under which conditions does CH₄ have the same number of molecules as 100 cm³ of O₂ at 27 °C and 1.0 x 10⁵ Pa?
C. Al₂O₃
Which represents an empirical formula?
A. C₂H₄
B. B₂H₆
C. Al₂O₃
D. C₆H₆
D
What are the coefficients of H₂SO₄(aq) and H₃PO₄(aq) when the following equation is balanced using the smallest possible whole numbers?
__Ca₃(PO₄)₂(s) + __H₂SO₄(aq) → __CaSO₄(s) + __H₃PO₄(aq)
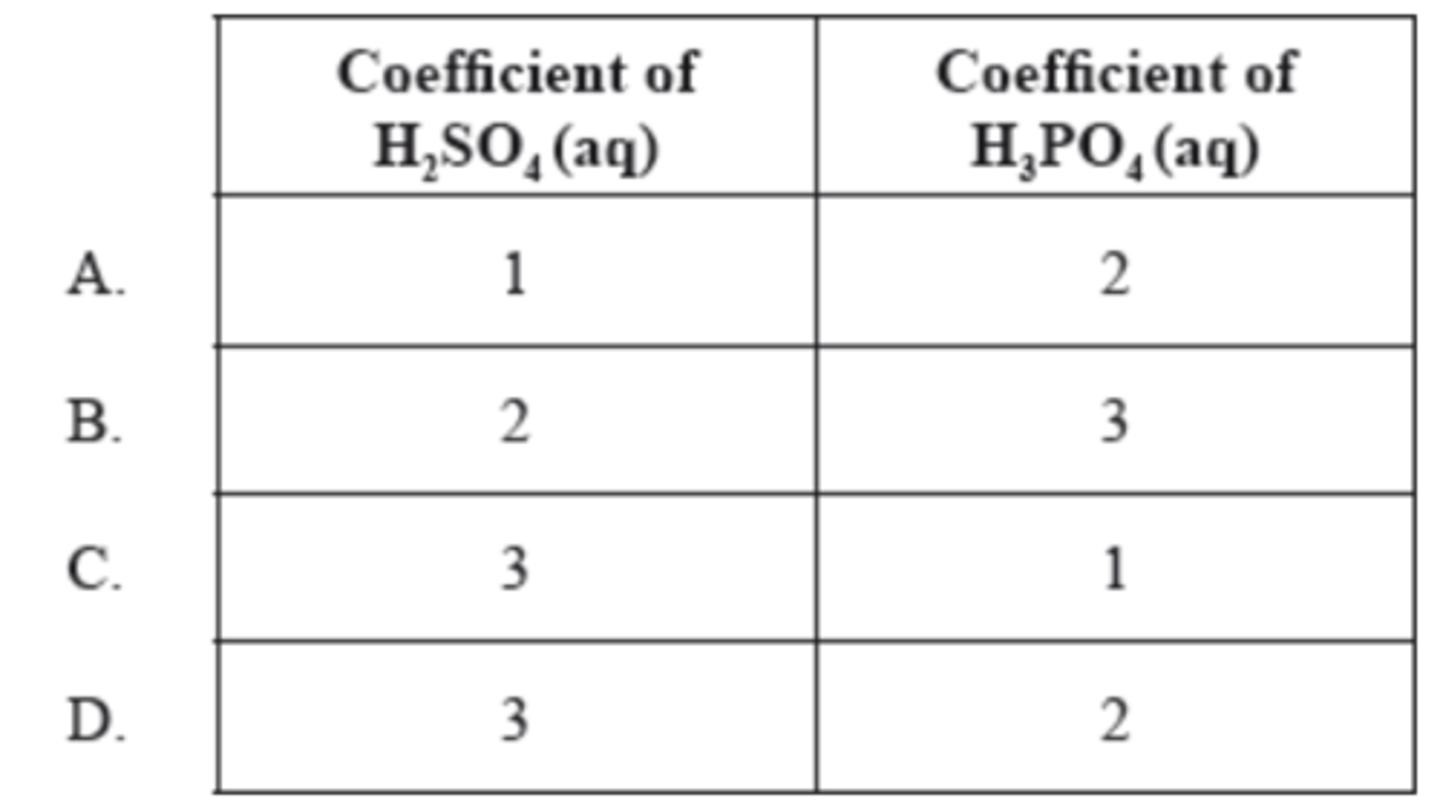
D
What is the pressure, in Pa, if 3 mol of gas occupies 500 cm³ at 25 °C?
Given: R = 8.31 J K⁻¹ mol⁻¹
10⁻³ m³ = 10³ cm³

B. 1.000 x 10⁻¹
7.102 g of Na₂SO₄ (M = 142.04 g mol⁻¹) is dissolved in water to prepare 0.5000 dm³ of solution. What is the concentration of Na₂SO₄ in mol dm⁻³?
A. 2.500 x 10⁻²
B. 1.000 x 10⁻¹
C. 1.000 x 10
D. 1.000 x 10²
D. I, II and III
Which statements are correct about Avogadro's constant?
I. It is the number of ions in 12 g of sodium hydride, NaH.
II. It is the number of molecules in 22.4 dm³ of hydrogen gas at 0 °C and 1 atm.
III. It is the number of atoms in 12 g of ¹²C.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
D. 60
What is the molar mass, in g mol⁻¹, of a substance if 0.30 mol of the substance has a mass of 18 g?
A. 5.4
B. 6.0
C. 30
D. 60
C. 4:5
What is the whole number ratio of the coefficients of ammonia to oxygen when the following equation is balanced correctly?
__NH₃(g) + __O₂(g) → __NO(g) + __H₂O(l)
A. 1:2
B. 2:1
C. 4:5
D. 5:4
D. C₄H₁₀
When 50 cm³ of a hydrocarbon, CxHy, was burned in excess oxygen, 200 cm³ of carbon dioxide and 250 cm³ of steam were produced (all volumes were measured under the same conditions). What is the molecular formula of the hydrocarbon?
A. C₂H₄
B. C₃H₈
C. C₄H₈
D. C₄H₁₀
C
What is the pressure, in Pa, in a 100 cm³ container containing 1.8 g of steam at a temperature of 727 °C? (R = 8.31 J K⁻¹ mol⁻¹)
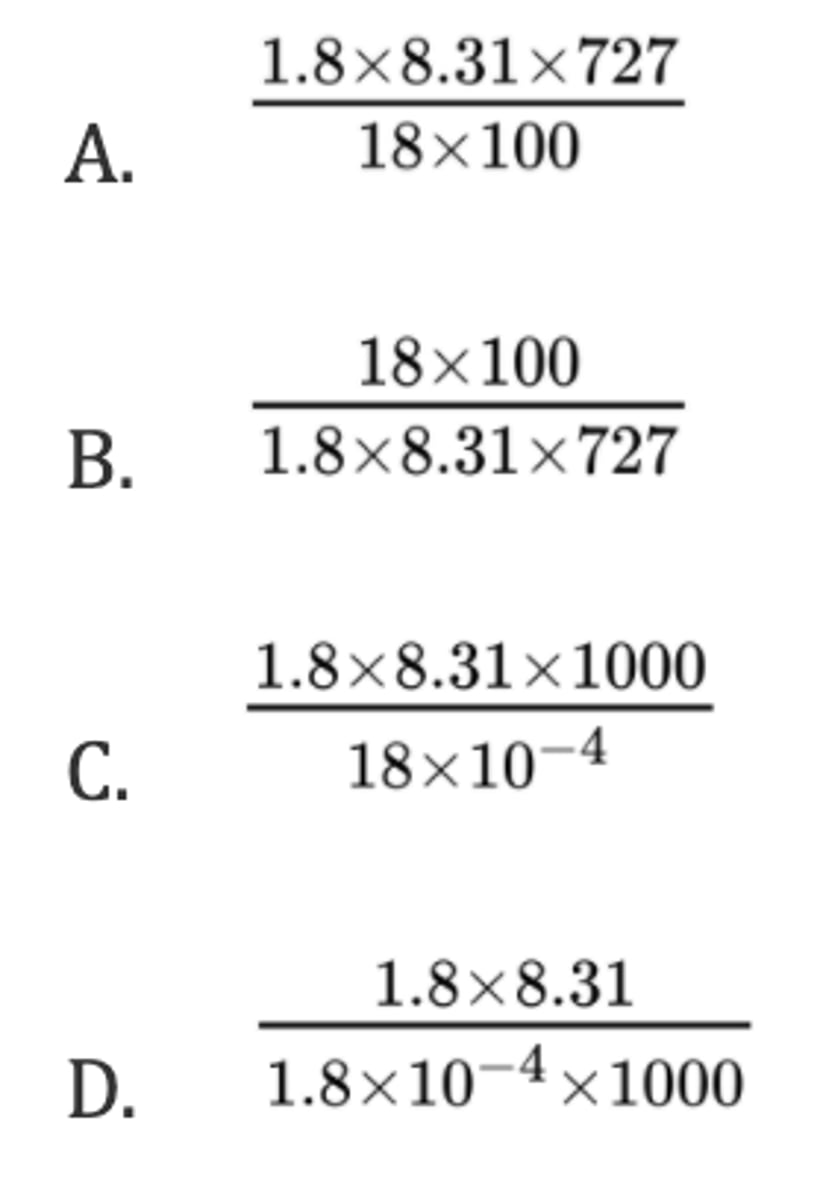
C. 2 mol of K₃PO₄
Which contains the largest number of ions?
A. 1 mol of Al₂(SO₄)₃
B. 1 mol of Mg₃(PO₄)₂
C. 2 mol of K₃PO₄
D. 3 mol of NaNO₃
D. The weighted mean mass of naturally occurring isotopes of an element compared to 1/12th of the mass of an atom of carbon-12
Which is the best description of relative atomic mass, Ar?
A. The number of neutrons and protons present in the nucleus of an atom
B. The average number of neutrons and protons in all isotopes of an element
C. The weighted mean mass of naturally occurring isotopes of an element compared to the mass of an atom of carbon-12
D. The weighted mean mass of naturally occurring isotopes of an element compared to 1/12th of the mass of an atom of carbon-12
B. 2.2
What mass of carbon dioxide, CO₂(g), in g, is produced when 5.0 g of calcium carbonate, CaCO₃(s),reacts completely with hydrochloric acid, HCl(aq)?
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) +CO₂(g)
A. 0.050
B. 2.2
C. 4.4
D. 5.0
C. 8
What volume of carbon dioxide, CO₂(g), in dm³, is produced when 1 dm³ of octane, C₈H₁₈(g), undergoes complete combustion?
2C₈H₁₈(g) + 25O₂(g) → 16CO₂(g) + 18H₂O(g)
A. 1
B. 4
C. 8
D. 9
D
What are the coefficients of H₂SO₄(aq) and H₃PO₄(aq) when the following equation is balanced using the smallest possible whole numbers?
__Ca₃(PO₄)₂(s) + __H₂SO₄(aq) → __CaSO₃(s) + __H₃PO₄(aq)
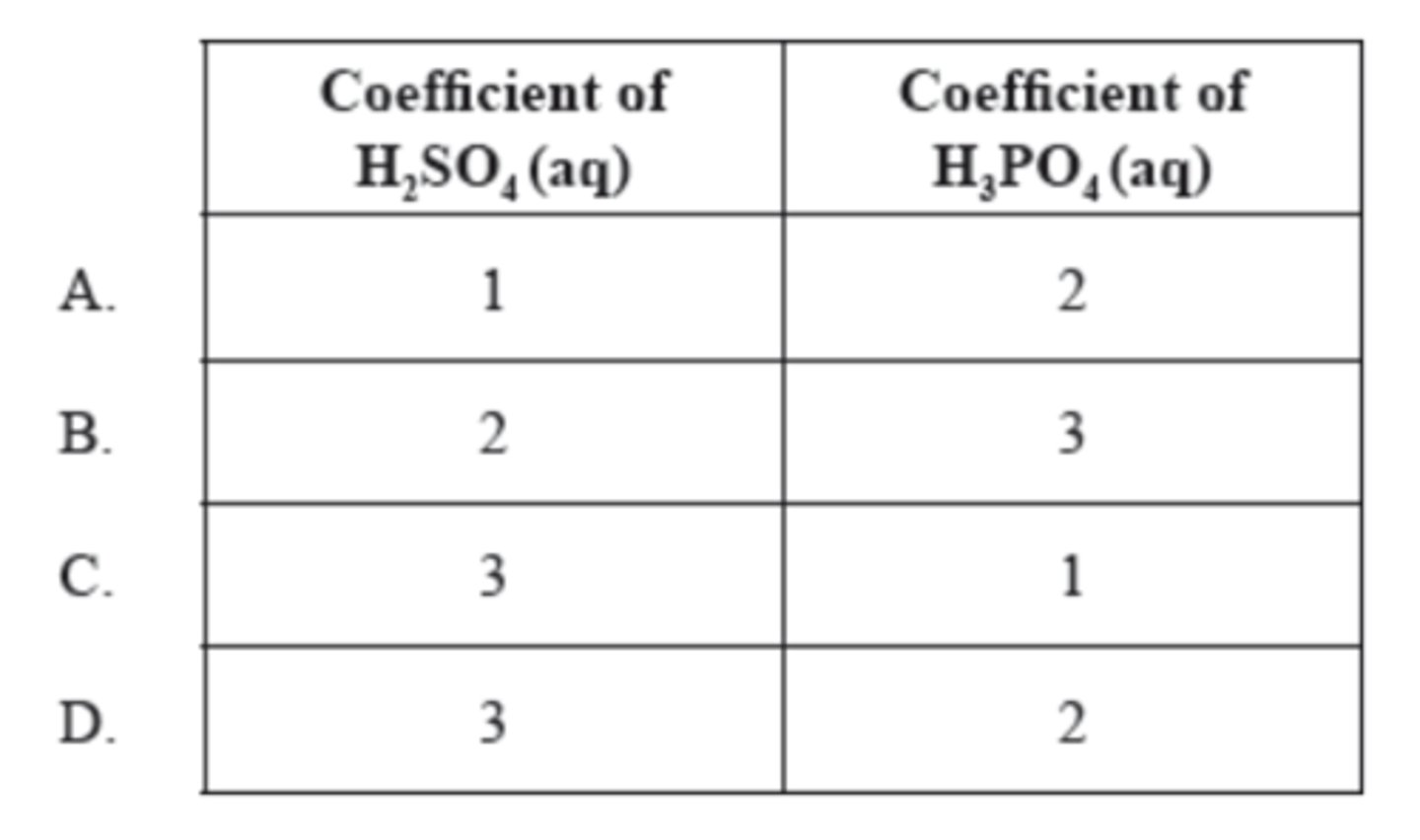
B. 1.000 x 10⁻¹
7.102 g of Na₂SO₄ (M = 142.04 g mol⁻¹) is dissolved in water to prepare 0.5000 dm³ of solution. What is the concentration of Na₂SO₄ in mol dm⁻³?
A. 2.500 x 10⁻²
B. 1.000 x 10⁻¹
C. 1.000 x 10
D. 1.000 x 10²
D
Which graph represents the relationship between volume and pressure for a fixed mass of gas at constant temperature?
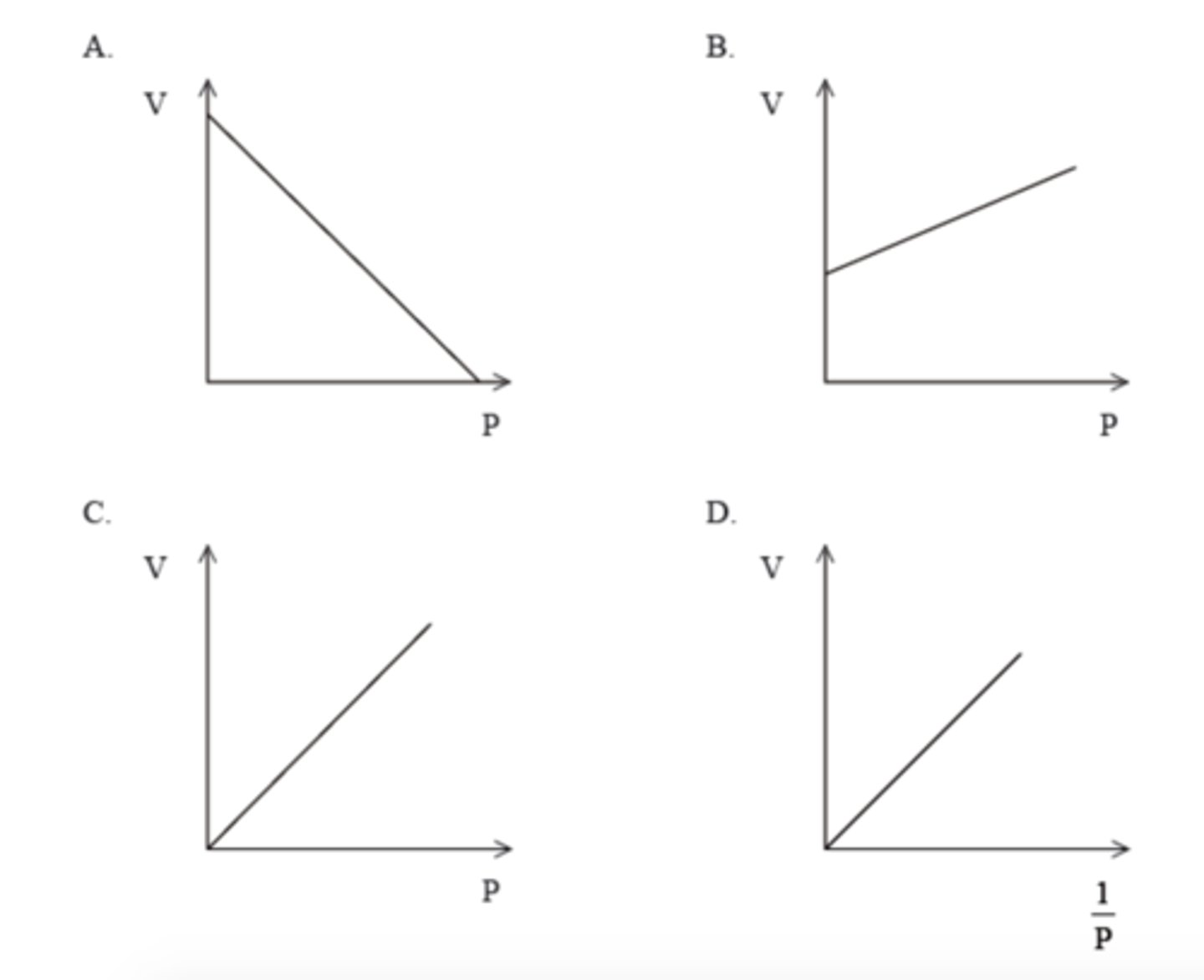
C. 4.8 x 10²³
What is the number of ions in 0.20 mol of (NH₄)₃PO₄?
A. 8.0 x 10⁻¹
B. 1.2 x 10²³
C. 4.8 x 10²³
D. 2.4 x 10²⁴
D. 286.19
What is the molar mass, in g mol⁻¹, of washing soda crystals, Na₂CO₃•10H₂O?
A. 105.99
B. 124.00
C. 263.15
D. 286.19
B. 66
The equation for the reduction of iron(III) oxide is:
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
What mass of carbon dioxide, in g, is produced by the complete reduction of 80 g of iron(III) oxide?
A. 44
B. 66
C. 88
D. 132
A. 1.5
3.0 dm³ of ethyne, C₂H₂, is mixed with of hydrogen and ignited. The equation for the reaction that occurs is shown below.
C₂H₂(g) + 2H₂(g) → C₂H₆(g)
Assuming the reaction goes to completion and all gas volumes are measured at the same temperature and pressure, what volume of ethane, C₂H₆, in dm³, is formed?
A. 1.5
B. 2.0
C. 3.0
D. 6.0
D. 6.62 x 10²³
What is the total number of atoms in 0.100 mol of [Pt(NH₃)₂Cl₂]?
A. 11
B. 6.02 x 10²²
C. 3.01 x 10²³
D. 6.62 x 10²³
B. 4
Nitroglycerine, C₃H₅N₃O₉, can be used in the manufacture of explosives. What is the coefficient of C₃H₅N₃O₉(l) when the equation for its decomposition reaction is balanced using the lowest whole numbers?
__C₃H₅N₃O₉(l) → __CO₂(g) + __H₂O(l) + __N₂(g) + __O₂(g)
A. 2
B. 4
C. 20
D. 33
A. 2.24
The volume occupied by one mole of an ideal gas at 273 K and 1.01 x 10⁵ Pa is 22.4 dm³. What volume, in dm³, is occupied by 3.20 g O₂(g) at 273 K and 1.01 x 10⁵ Pa?
A. 2.24
B. 4.48
C. 22.4
D. 71.7
C
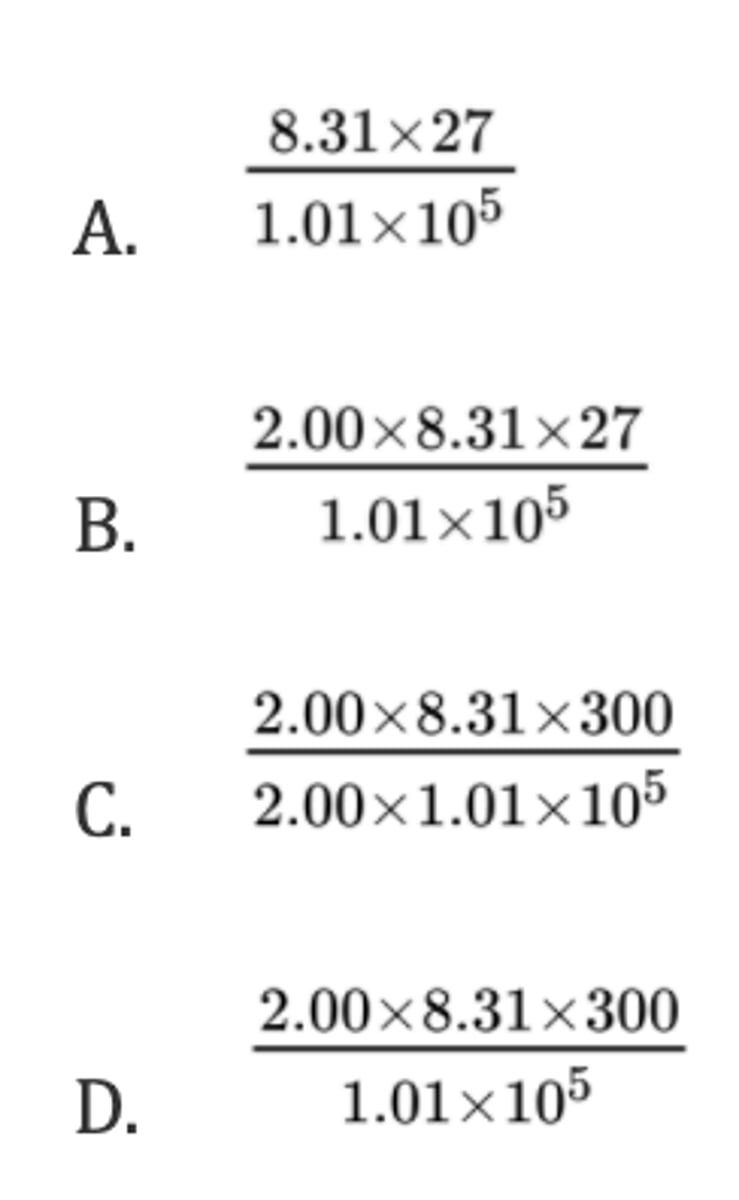
What volume, in m³, is occupied by 2.00 mol of gas at 27 °C and 2.00 atm pressure?
Assume: 1.00 atm = 1.01 x 10⁵ Pa and R = 8.31 J K⁻¹ mol⁻¹.
D. I, II and III
Which statements about solutions are correct?
I. A solute dissolves in a solvent to form a solution.
II. A solution is a homogeneous mixture of two or more substances.
III. Concentrations of solutions can be expressed in g dm⁻³.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
D. 9
What is the sum of the coefficients when the following equation is balanced using whole numbers?
__Fe₂O₃(s) + __CO(g) → __Fe(s) + __CO₂(g)
A. 5
B. 6
C. 8
D. 9
C. 1.1
1.0 dm³ of an ideal gas at 100 kPa and 25 °C is heated to 50 °C at constant pressure. What is the new volume in dm³?
A. 0.50
B. 0.90
C. 1.1
D. 2.0
A. 2.0 x 10⁻³
What is the amount, in moles, of sulfate ions in 100 cm³ of 0.020 mol dm⁻³ FeSO₄(aq)?
A. 2.0 x 10⁻³
B. 2.0 x 10⁻²
C. 2.0 x 10⁻¹
D. 2.0
D. 13
What is the sum of all coefficients when the following equation is balanced using the smallest possible whole numbers?
__C₂H₂ + __O₂ → __CO₂ + __H₂O
A. 5
B. 7
C. 11
D. 13
B. 0.1
1.7 g of NaNO₃ (Mr = 85) is dissolved in water to prepare 0.20 dm³ of solution. What is the concentration of the resulting solution in mol dm⁻³?
A. 0.01
B. 0.1
C. 0.2
D. 1.0
A. 3.0 x 10¹⁹
How many molecules are present in a drop of ethanol, C₂H₅OH, of mass 2.3 x 10⁻³ g?
(L = 6.0 x 10²³ mol⁻¹)
A. 3.0 x 10¹⁹
B. 3.0 x 10²⁰
C. 6.0 x 10²⁰
D. 6.0 x 10²⁶
B. 2 mol of N₂O
Which sample has the greatest mass?
A. 1 mol of SO₂
B. 2 mol of N₂O
C. 2 mol of Ar
D. 4 mol of NH₃
D. C₄H₈
The relative molecular mass of a gas is 56 and its empirical formula is CH₂. What is the molecular formula of the gas?
A. CH₂
B. C₂H₄
C. C₃H₆
D. C₄H₈
D
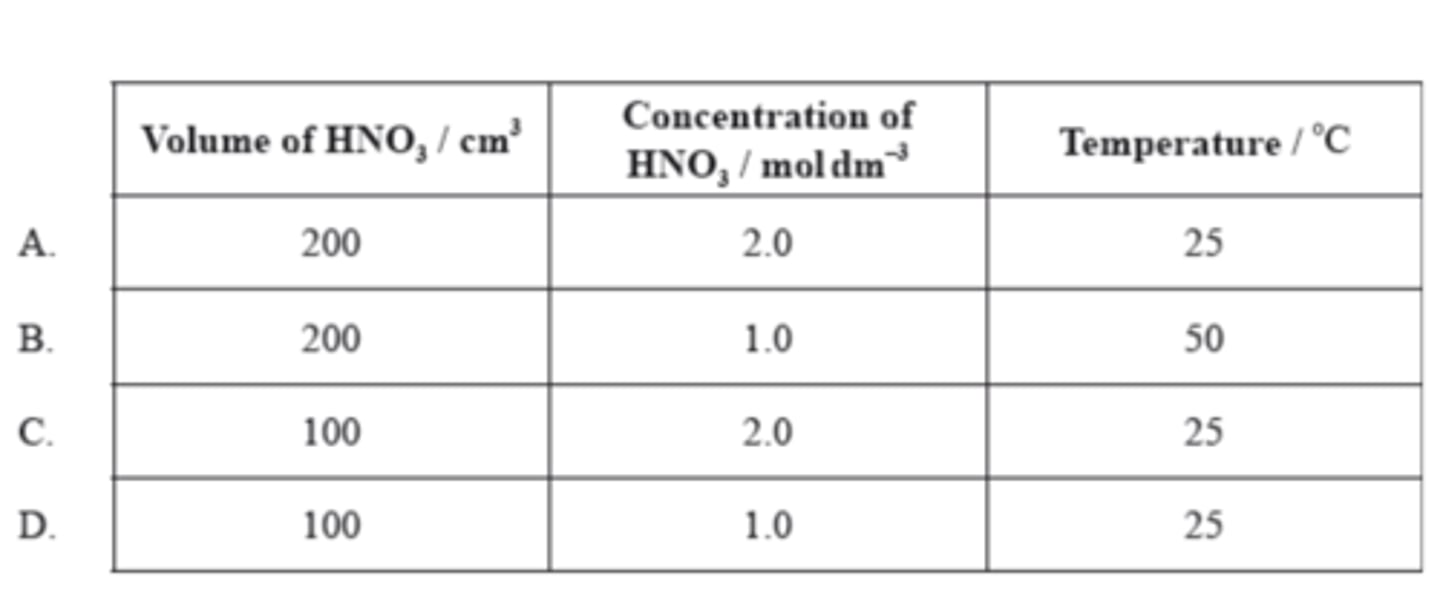
At 25 °C, 200 cm³ of 1.0 mol dm⁻³ nitric acid is added to 5.0 g of magnesium powder. If the experiment is repeated using the same mass of magnesium powder, which conditions will result in the same initial reaction rate?
D. 8
What is the sum of the coefficients for the equation when balanced using the smallest possible whole numbers?
__N₂H₄(g) + __O₂(g) → __NO₂(g) + __H₂O(g)
A. 5
B. 6
C. 7
D. 8
A. 4.00
Chloroethene, C₂H₃Cl, reacts with oxygen according to the equation below.
2C₂H₃Cl(g) + 5O₂(g) → 4CO₂(g) + 2H₂O(g) + 2HCl(g)
What is the amount, in mol, of H₂O produced when 10.0 mol of C₂H₃Cl and 10.0 mol of O₂ are mixed together, and the above reaction goes to completion?
A. 4.00
B. 8.00
C. 10.0
D. 20.0
D. 646 K
A fixed mass of gas has a certain volume at a temperature of 50 °C. What temperature is required to double its volume while keeping the pressure constant?
A. 100 K
B. 323 K
C. 373 K
D. 646 K