Biomolecules
1/53
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
54 Terms
Biomolecules
Chemical compounds found in living organisms.
Responsible for their growth, maintenance and ability to reproduce.
Examples:
Carbohydrates
Proteins
Nucleic acids
Lipids
Enzymes
Vitamins
Hormones
Carbohydrates or Saccharides
These are the hydrates of carbon and most of them have a general formula Cₓ(H₂O)ᵧ (Old definition)
Polyhydroxy aldehydes or ketones or the compounds which produce carbohydrates on hydrolysis. (Modern definition) e.g.: glucose, sucrose, cellulose, starch etc.
Optically active compounds
Classification of Carbohydrates
Based on their behaviour on hydrolysis
Based on reducing character
Based on their physical properties
Based on Their Behavior on Hydrolysis
Monosaccharides
Simplest carbohydrates which cannot be further hydrolysed
Eg: Glucose, Fructose, Galactose, Ribose etc
Oligosaccharides
Carbohydrates which give 2-10 units of monosaccharides on hydrolysis
Eg: Sucrose, Maltose, Lactose
This can be further classified into:
Disaccharides:
Sucrose (hydrolysed into Glucose + Fructose)
Maltose (hydrolysed into 2 molecules of Glucose)
Lactose (hydrolysed into Glucose + Galactose)
Rafinose (hydrolysed into Glucose + Fructose + Galactose)
Trisaccharides
Tetrasaccharides
Polysaccharides
Carbohydrates which give a large number of monosaccharide units on hydrolysis.
Eg: Starch, Cellulose, Glycogen, Gums etc.
Invert Sugar
Cane sugar is sucrose, which on hydrolysis gives an equimolar mixture of D(+)glucose and D(-)fructose.
C₁₂H₂₂O₁₁ + H₂O → C₆H₁₂O₆ (D(+)Glucose, +52.5°) + C₆H₁₂O₆ (D(-)Fructose, -92.4°)
Sucrose is dextro rotatory but after hydrolysis gives dextro rotatory glucose and laevo rotatory fructose. Since the laevo rotation of fructose (-92.40°) is more than the dextro rotation of glucose (+52.50°), the mixture is laevo rotatory.
Thus, hydrolysis of sucrose brings about a change in the sign of rotation from dextro (+) to laevo (-), and the product is named as invert sugar. The process is called inversion of cane sugar.
Based on Reducing Character
Reducing Sugars
Carbohydrates which contain free aldehydic or ketonic group and reduce Fehling’s Solution and Tollen’s Reagent
Eg: All monosaccharides, disaccharides like maltose and lactose
Non-Reducing Sugars
Carbohydrates which do not have an aldehydic or ketonic group and do not reduce Fehling’s Solution and Tollen’s Reagent
Eg: Sucrose, all polysaccharides
Based on Their Physical Properties
Sugars
Carbohydrates which are sweet in taste, crystalline and water soluble.
Eg: all monosaccharides and disaccharides
Non-Sugars
Carbohydrates which have no sweet taste, not crystalline and water insoluble.
Eg: all polysaccharides
Monosaccharides Classification
Based on Functional Group
Aldose: Monosaccharide containing an aldehyde (-CHO) group.
Ketose: Monosaccharide containing a keto (>C = 0) group.
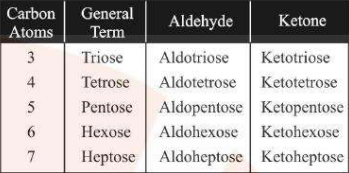
Based on the Number of Carbon Atoms
Monosaccharides containing 3 carbon atoms are called triose, 4 carbon atoms are called tetrose etc. (see image)
Glucose
Glucose is an aldohexose
Molecular formula is C6H1206
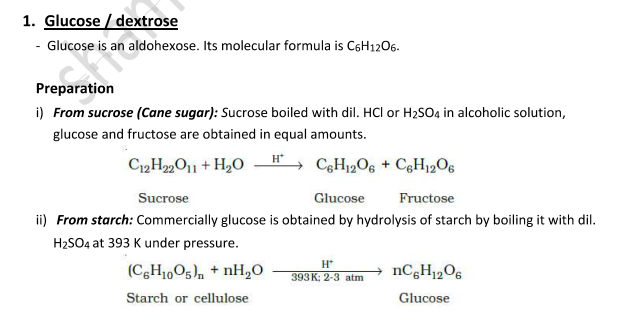
Reactions of Glucose
How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
When glucose reacts with different reagents, the following products are formed along with key inferences:
With HI:
Product: n-Hexane
Inference: All the 6 carbon atoms are linked in a straight chain.
With HCN:
Product: Cyanohydrin
Inference: Indicates the presence of a carbonyl group.
With NH₂OH:
Product: Oxime
Inference: Confirms the presence of a carbonyl group.
A: Pentaacetate of D-glucose in aqueous medium does not form an open chain structure and thus when it reacts with NH₂OH, it does not form oxime indicating that a free aldehyde (–CHO) group is absent.
With Bromine water (Br₂/H₂O):
Product: Gluconic acid
Inference: This shows that the carbonyl group is an aldehyde.
With Acetic anhydride:
Product: Glucose pentaacetate
Inference: Indicates the presence of five –OH groups.
With Conc. HNO₃:
Product: Saccharic acid
Inference: Confirms that one of the –OH groups is primary.
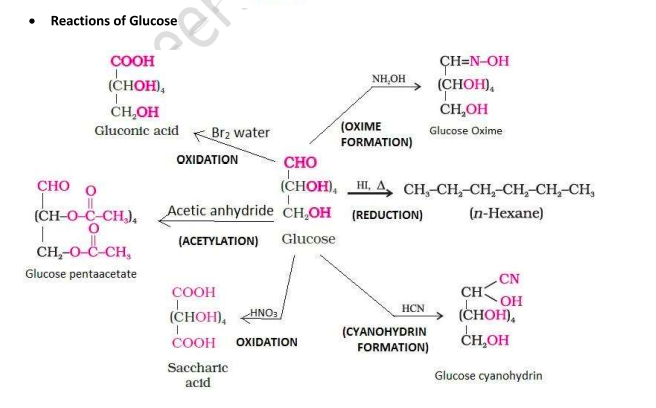
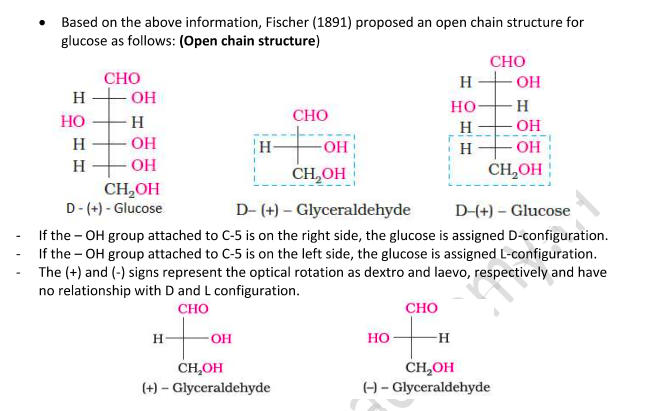
Open Chain Structure of Glucose
But this open chain structure cannot explain the following observations:
Glucose does not react with 2,4-Dinitrophenyl hydrazine, Schiff’s reagent, and with NaHSO₃.
The pentaacetate of glucose does not react with hydroxylamine, indicating the absence of a free —CHO group.
The existence of two different crystalline forms of glucose - (α and β form).
In order to explain the above, it was proposed that one of the —OH groups may add to the —CHO group and form a cyclic hemi-acetal structure. The —OH at C₅ is involved in ring formation (1,5-oxide ring).
Thus, the two cyclic forms exist in equilibrium with the open chain structure.
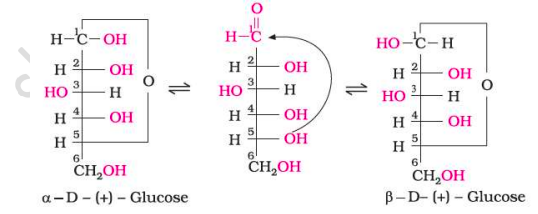
Anomers
They are stereoisomers which differ only in the configuration at the hemiacetal or hemiketal carbon.
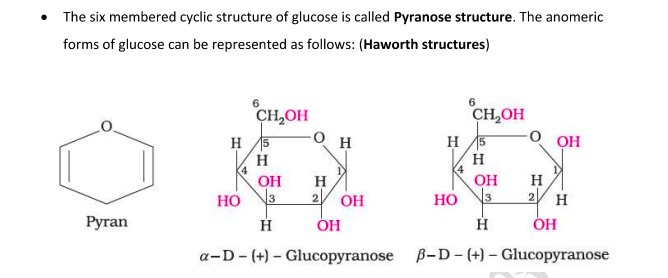
Pyranose Structure of Glucose
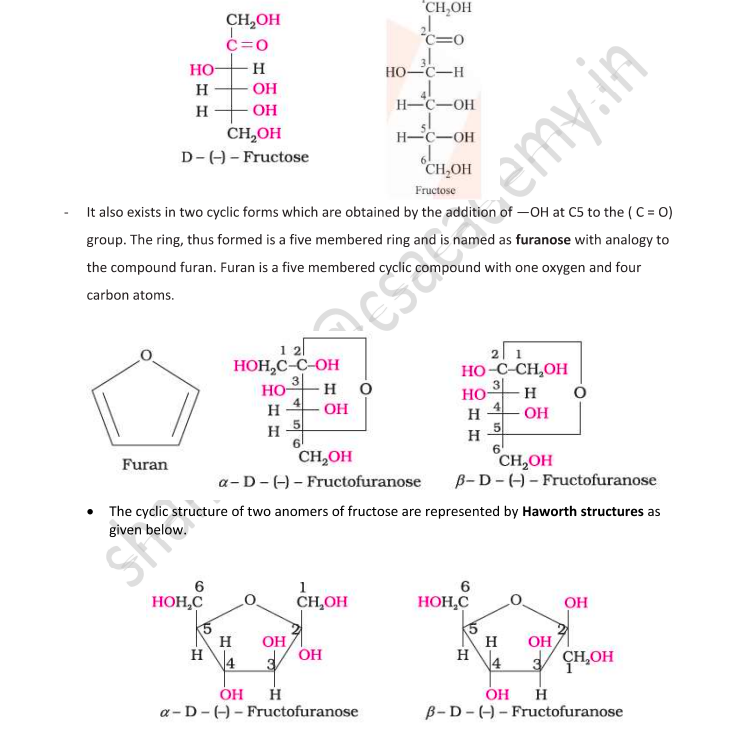
Fructose
Fructose is an important ketohexose also with the molecular formula C₆H₁₂O₆.
It belongs to the D-series and is a laevorotatory compound, and it is written as D-(-)-Fructose.
Reactions show that fructose has a ketonic group at the second carbon.
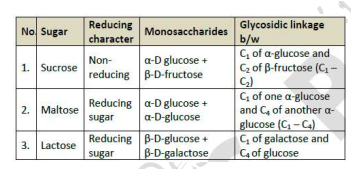
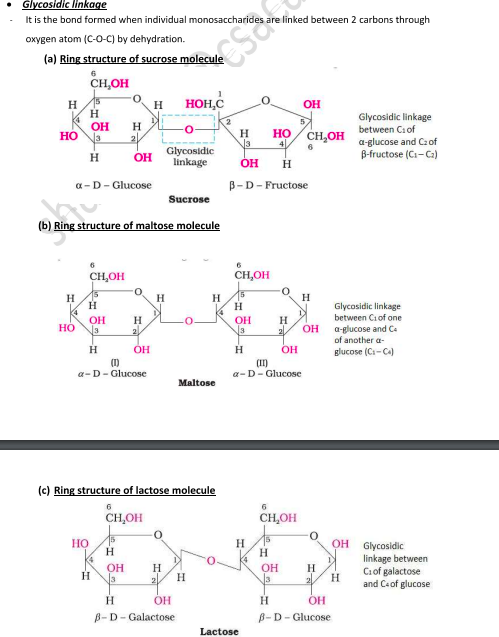
Gycosidic Linkage
Assertion (A) β-glycosidic linkage is present in maltose.
Reason (R) Maltose is composed of two glucose units in which C-1 of one glucose unit is linked to C-4 of another glucose unit.
The oxide linkage formed by the loss of a water molecule when two monosaccharides are joined together through oxygen atom is called glycosidic linkage.
A: d) A is false, R is true
since the linkage is between two alpha molecules not beta
Polysaccharides
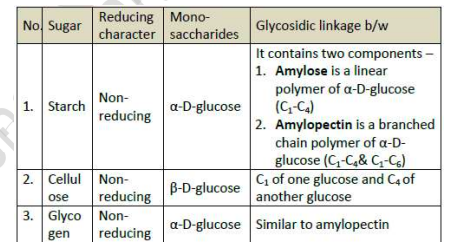
Starch
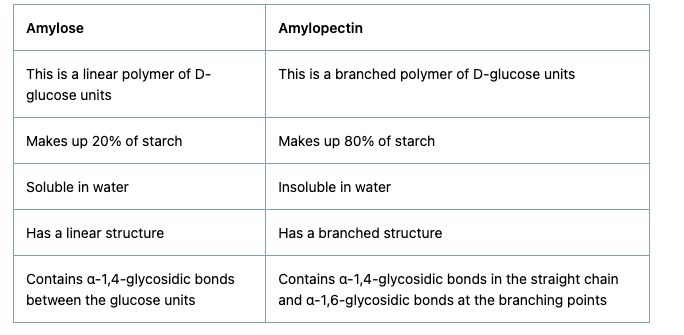
It is a polymer of α -glucose and consists of two components — Amylose and Amylopectin
Amylose vs Amylopectin
Write one structural differences between Amylose and Amylopectin
Amylose: (remember as amy-less so contributes lesser)
Water soluble component
Long unbranched chain polymer
It contains 200 – 1000 α -D-(+)- glucose units held by α - glycosidic linkages involving C1 – C4 glycosidic linkage
Constitutes to about 15-20% of starch
Amylopectin:
Water insoluble component
Branched chain polymer
It forms chain by C1 – C4 glycosidic linkage whereas branching occurs by C1 – C6 glycosidic linkage
It constitutes about 80-85% of starch
Cellulose
It is a polysaccharide composed only of β -D-glucose units which are joined by glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
Glycogen
The carbohydrates are stored in animal body as glycogen
Also known as animal starch because its structure is similar to Amylopectin
It is present in liver, muscles and brain
Enzymes break it down into glucose when the body needs it
It is also found in yeast and fungi
Amino acids
Amino acids contain amino (–NH2) and carboxyl (–COOH) functional groups
Types of Amino Acids
Essential Amino Acids
Cannot be synthesized by our body
Should be taken in our diet
Eg: Valine, Leucine
Non-Essential Amino Acids
Can be synthesized by our body
Does not need to be taken in our diet
Eg: Glycine, Alanine
Note:
Except glycine, all other naturally occurring α-amino acids are optically active, since the α-carbon atom is asymmetric. These exist both in ‘D’ and ‘L’ forms.
Most naturally occurring amino acids have L-configuration. L-Amino acids are represented by writing the –NH₂ group on the left-hand side.
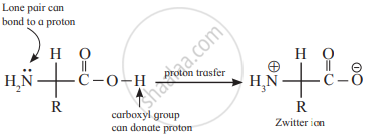
Amphoteric Nature/Zwitter ion form of amino acids
Amino acids act as salts rather than simple amines or carboxylic acids
This is due to the presence of both basic (amino) and an acidic (carboxyl) group
In aqueous solutions, the carboxyl group can lose a proton and the amino group can gain a proton giving rise to a dipolar ion known as zwitter ion
They are neutral but contain both positive and negative charges
In zwitter ionic form, amino acids show amphoteric behaviour as they react both with
acids and bases
Isoelectronic point
The pH of the solution at which the dipolar ion exists as a neutral ion and does not migrate to either electrode
Proteins
Polymers of β-amino acids and they are connected to each other by peptide linkage
Peptide Linkage
Peptide linkage is an amide linkage formed by condensation reaction between –COOH group of one amino acid and –NH2 group of another amino acid
Functional vs Structural Proteins
Fibrous/Structural Proteins:
Responsible for the structure of the body
Water insoluble
Polypeptide chains run parallel and are held together by hydrogen and di-sulphide bonds
Eg: Keratin (hair), Myosin (Mucles)
Globular/Functional Proteins:
Responsible for the function of the body
Water soluble
Polypeptide chains coil around each other to give a spherical shape
Eg: Insulin, Albumins
Primary Structure of Proteins
The sequence of amino acids is said to be the primary structure of a protein.
Secondary Structure of Proteins
Refers to the shape in which a long polypeptide chain can exist
α-Helix
A protein thread is folded in the form of a helix. This structure is maintained by H-bonds which are formed between –NH group of one amino acid and –CO group 4 amino acids away. It has only right-handed helices.
β-Pleated Sheet
In this, the polypeptide chains lie side by side in a zig-zag manner with alternate R groups on the same side situated at fixed distances apart.
Tertiary Structure of Proteins
It represents the overall folding of the polypeptide chain i.e., further folding of the 2° structure. It gives rise to two major molecular shapes - fibrous and globular
Types of Bonding Which Stabilize the Structures
What type of linkage is responsible for the secondary structure of proteins?
1o
Peptide bonds
2o
Hydrogen bonds
Peptide linkage
3o
Disulphide bridge
H – bonding
Salt bridge
Hydrophobic interactions
van der Waals forces
Quaternary Structure of Proteins
Some proteins are composed of two or more polypeptide chains called sub-units.
The spatial arrangement of these subunits with respect to each other is known as quaternary structure of proteins.
Native Protein
Protein found in a biological system with a unique 3-D structure and biological activity
Denaturation of Proteins
The loss of biological activity of proteins when a protein in its native form, is subjected to physical change like a change in temperature or chemical change like a change in pH due to which globules unfold and the helix gets uncoiled and the protein loses its biological activity
This is called the denaturation of protein
During denaturation, secondary and tertiary structures are destroyed but the primary structure remains intact
Example: coagulation of egg white on boiling, curdling of milk
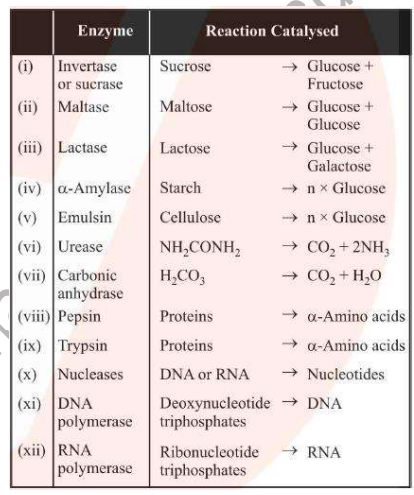
Enzymes
Biological catalysts which catalyse biochemical reactions in living beings
Highly selective and specific
Increase the speed of the reactions about 10M times
Extremely small quantities of enzymes (as small as 1 millionth of a mole) can increase the rate by a million times
Enzymes are active only at temperatures of about 37oC and pH of around 7
Commonly used enzymes: invertase, pepsin (protein to amino acids), amylase (starch to glucose), urease (urea to carbon dioxide and ammonia)
Vitamins
Organic compounds required in the diet in small amounts to perform specific biological functions for normal maintenance of optimum growth and health of the organisms
Classification of Vitamins
Why cannot vitamin C be stored in our body?
Fat Soluble Vitamins
Vitamins which are soluble in fat and oils but insoluble in water
They are stored in the liver and adipose (fat-storing) tissues
Eg: Vitamin A, D, E and K
Water Solutble Vitamins
Vitamins that are soluble in water
They must be supplied regularly in diet because they are readily excreted in urine and cannot be stored in our body
Eg: Vitamin B and C
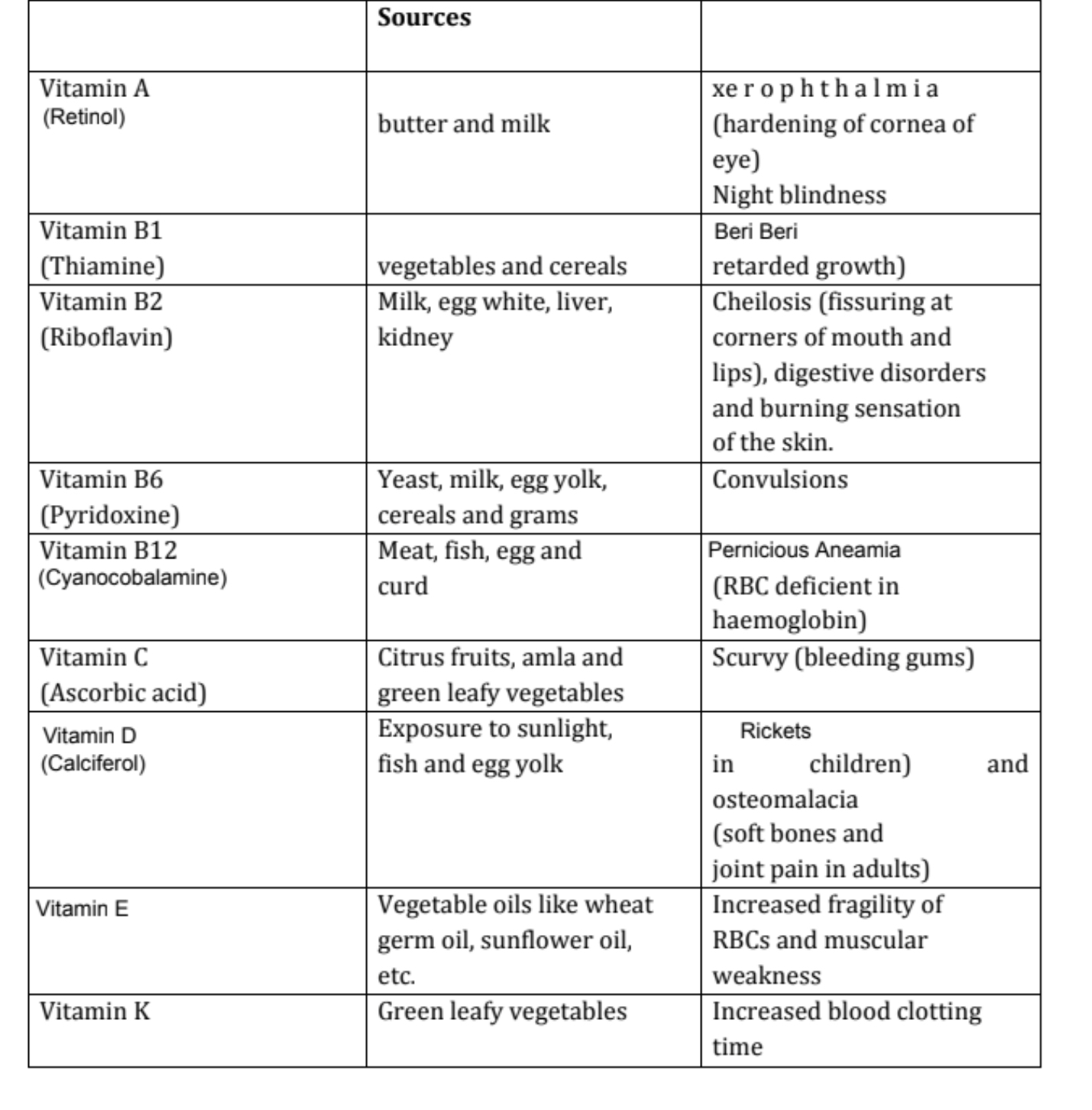
Vitamin A
Source:
Milk
Butter
Fish liver oil
Carrots
Deficiency Diseases:
Xerophthalmia (hardening of cornea of eye)
Night blindness
Vitamin B₁ (Thiamine)
Source:
Milk
Yeast
Cereals
Green vegetables
Deficiency Diseases:
Beri Beri (loss of appetite and retarded growth)
Vitamin B₂
Source:
Milk
Egg white
Liver
Kidney
Deficiency Diseases:
Cheilosis (fissuring at corners of mouth and lips)
Digestive disorders
Burning sensation of skin
Vitamin B₆ (Pyridoxine)
Source:
Milk
Yeast
Egg yolk
Cereals
Grams
Deficiency Diseases:
Convulsions
Vitamin B₁₂
Source:
Curd
Meat
Fish
Egg
Deficiency Diseases:
Pernicious anaemia (RBC deficient in haemoglobin)
Vitamin C (Ascorbic Acid)
Source:
Citrus fruits
Amla
Green leafy vegetables
Deficiency Diseases:
Scurvy (bleeding gums)
Vitamin D
Source:
Sunlight
Fish
Egg yolk
Deficiency Diseases:
Rickets (bone deformities in children)
Osteo-malacia (soft bones and joint pain in adults)
Vitamin E
Source:
Vegetable oils like:
Germ oil
Sunflower oil
Deficiency Diseases:
Increased fragilitiy of RBCs and muscular weakness
Vitamin K
Source:
Green leafy vegetables
Deficiency Diseases:
Increased blood clotting time
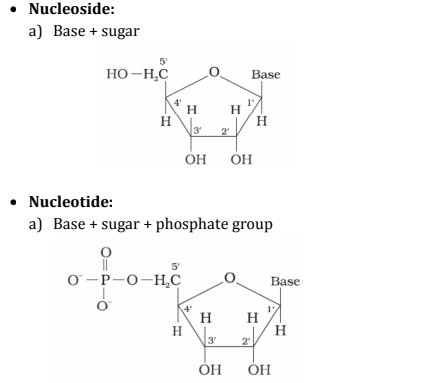
Nucleoside and Nucleotide
Nucleoside: A unit formed by the combination of pentose sugar with the base is known as nucleoside.
(Base is attached to the 1st position of sugar)Nucleotide: When nucleoside combines with the phosphoric acid group, the unit obtained is called nucleotide.
(Phosphoric acid attached to the 5th position of sugar)
Phosphodiester Linkage
The Phosphester bond is a type of covalent bond that forms between a phosphate and a hydroxyl group (-OH)
In the case of nucleic acids, the phosphate group forms an ester with the hydroxyl groups on the 5th carbon of one of the nucleotides and the 3rd carbon of another nucleotide.
As the phosphate forms two ester bonds (double ester), hence the name phosphodiester linkage
This bond is formed due to the condensation reaction occurring between the hydroxyl group of two sugar groups and one phosphate group
Nucleic Acids
They are long-chain polymers of nucleotides
Found in the nuclei of all living cells in the form of nucleoproteins or chromosomes
Play an important role in the transmission of hereditary characteristics and the biosynthesis of proteins
Types of Nucleic Acids (DNA vs RNA)
Write two structural differences between DNA and RNA
DNA:
Responsible for the transfer of hereditary characteristics from one generation to another in living organisms. This is because of the unique property of replication during cell division and the transfer of two identical DNA strands to the daughter cells.
Contains deoxyribose pentose sugar
Contains four nitrogenous bases: Adenine (A), Guanine (G), Cytosine (C) and Thymine (T)
Has a double-stranded helical structure
RNA
Responsible for the synthesis of proteins
Contains ribose pentose sugar
Contains four nitrogenous bases: Adenine (A), Guanine (G), Cytosine (C) and Uracil (U)
Has a single-stranded helical structure
Types of RNA
i) Ribosomal RNA (rRNA)
ii) Messenger RNA (mRNA)
iii) Transfer RNA (tRNA)
Nitrogenous Bases Present in Nucleic Acids
Nucleic acid contains two types Nitrogen containing bases:
Purines: There are two bases derived from purines - Adenine (A) and Guanine (G)
Pyrimidines: There are three bases derived from pyrimidines - Thymine (T), Cytosine (C) & Uracil
In a DNA molecule, the nitrogenous bases are present as a complimentary base pair
Adenine always combines with Thyamine through a double hydrogen bond
Cytosine always combines with Guanine with a triple hydrogen bond to make up that double strand chain of a DNA molecule
Hydrolysis of Nucleic Acids
Generally nucleic acids (both DNA & RNA) upon hydrolysis give …
Ans: Complete hydrolysis of nucleic acids yields a pentose sugar, phosphoric acid, and nitrogen-containing heterocyclic compounds (Nitrogenous bases).What products would be formed when a nucleotide from DNA containing thymine is hydrolyzed?
Ans: When a nucleotide from the DNA containing thymine is hydrolyzed, thymine, β-D-2-deoxyribose, and phosphoric acid are obtained as products.
When RNA is hydrolyzed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Ans: When RNA is hydrolyzed, there is no relationship among the quantities of different bases obtained. This fact suggests that RNA is a single-strand structure, unlike DNA, which is a double-strand structure where pairing of bases occurs (e.g., adenine pairs with thymine).