Physics - P2 Thermal Physics
1/53
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
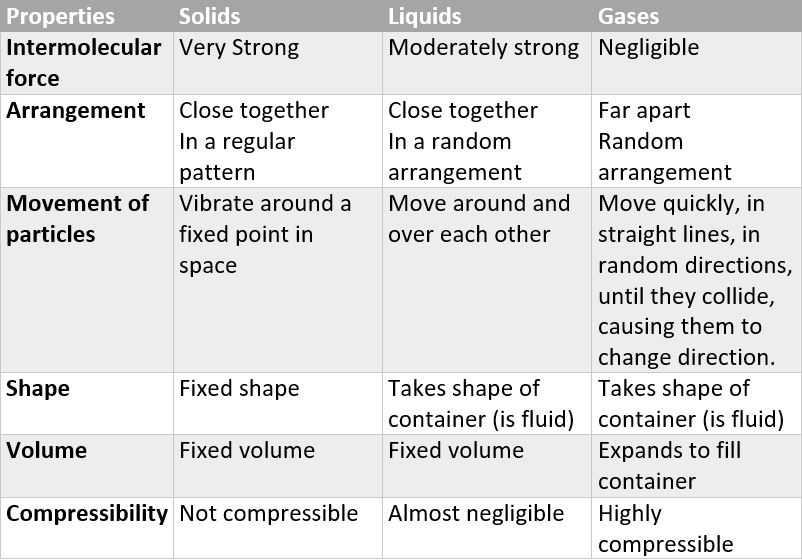
distinguishing properties of solids
rigid
fixed shape
fixed volume
cannot be compressed
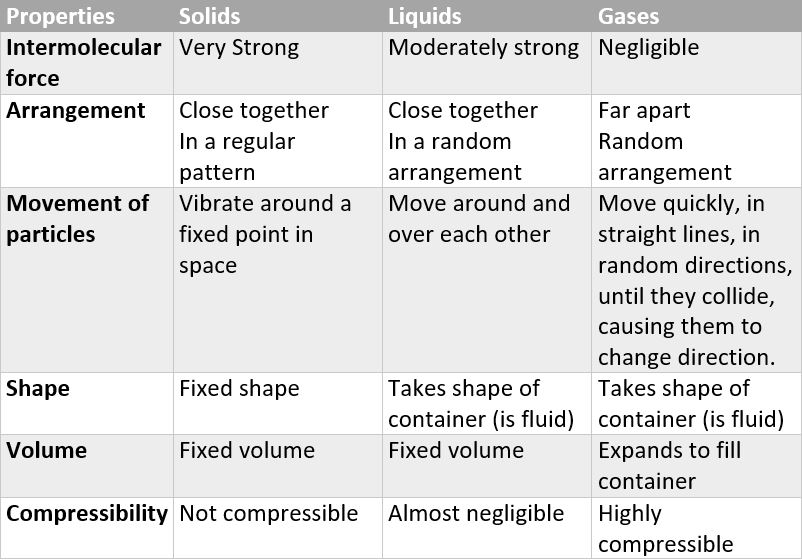
distinguishing properties of liquids
not rigid
no fixed shape
fixed volume
cannot be compressed
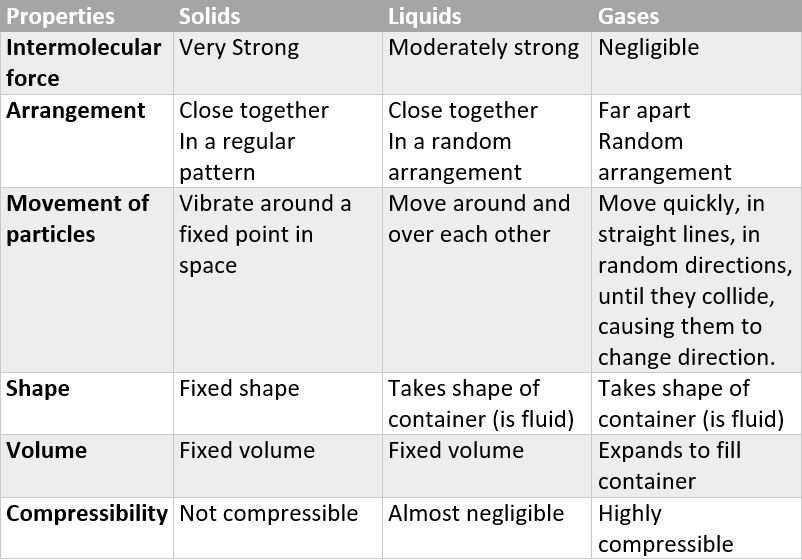
distinguishing properties of gases
not rigid
no fixed shape
no fixed volume
can be compressed
solid to liquid
melting
liquid to gas
evaporation
gas to liquid
condensation
liquid to solid
freezing/solidification
solids arrangement
Particles are closely packed: The particles in a solid are arranged in a regular, repeating pattern. This often forms a crystal lattice structure.
Fixed positions: Each particle vibrates around a fixed position, but it does not move from its place in the structure.
solids separation
Minimal separation: Particles are very close together, with very little space between them. The intermolecular forces are strong, holding the particles tightly in place.
solids motion
Vibrational motion: Particles in a solid vibrate but do not translate or move around. Their motion is restricted to small vibrations around their fixed positions.
solid liquid and gas particle diagram
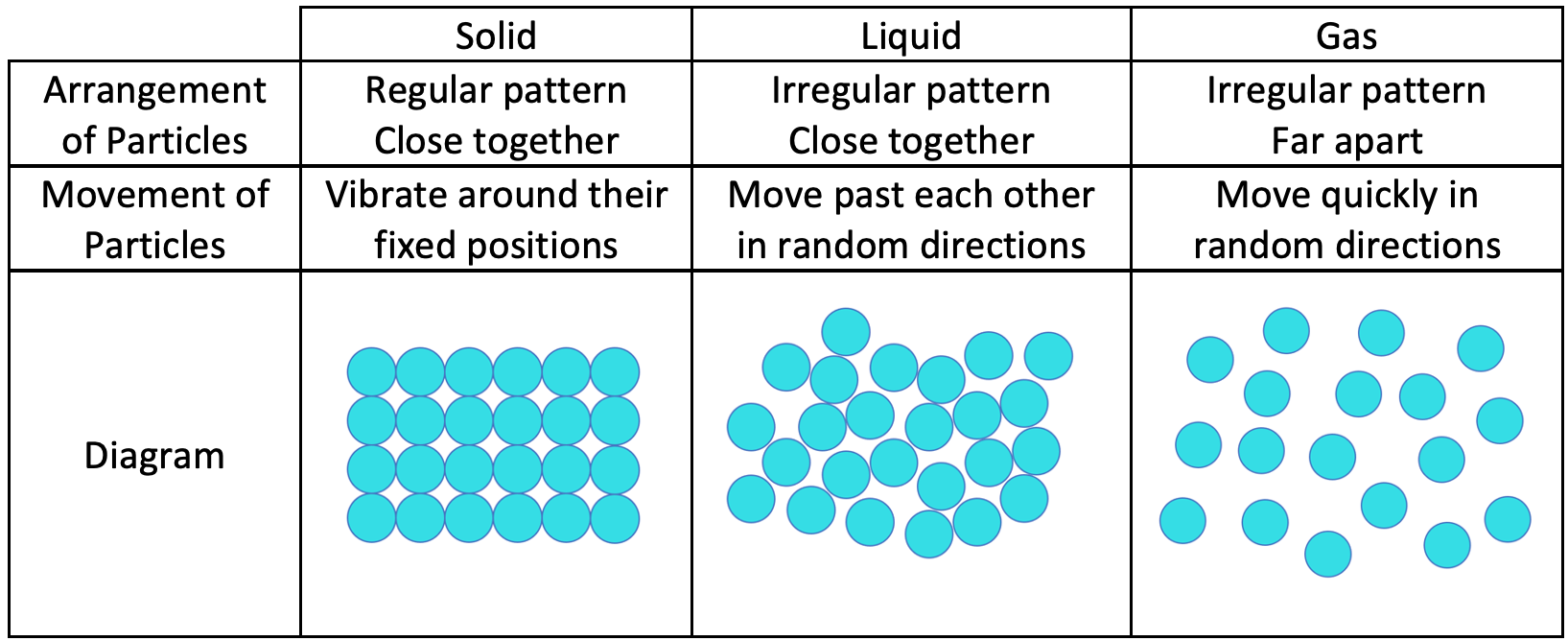
liquids arrangement
Close but irregular: Particles in a liquid are close together but not in a fixed, orderly pattern. They can slide past each other.
Some short-range order: While there is no long-range order like in solids, there may be some short-range ordering due to intermolecular attractions.
liquids separation
Moderate separation: Particles are still close, but there is more space between them than in solids. The intermolecular forces are weaker than in solids but still significant.
liquids motion
Translational motion: Particles can move past each other, allowing liquids to flow and take the shape of their container.
Vibrational and rotational motion: In addition to translational movement, particles can also vibrate and rotate.
gases arrangement
Very loose and random: Particles in a gas are far apart and move independently of each other. There is no regular arrangement.
Randomly distributed: The particles are distributed randomly throughout the container.
gases motion
Rapid and random motion: Particles move rapidly in all directions, colliding with each other and the walls of the container. Their motion is much more energetic than in solids or liquids.
Free movement: Particles travel in straight lines until they collide with something, changing their direction due to these collisions.
gases separation
Large separation: Particles are much farther apart than in solids and liquids. The intermolecular forces are negligible, allowing particles to move freely.
the forces and distances between particles and the motion of the particles affect…
the properties of solids, liquids and gases
At low temperatures
Particles move more slowly and have less kinetic energy. In solids, this means less vigorous vibrations. In liquids, particles move more sluggishly, and in gases, particles collide less energetically.
At high temperatures
Particles move more rapidly and have more kinetic energy. In solids, this leads to more vigorous vibrations, potentially causing melting. In liquids, particles move faster, which can lead to boiling. In gases, particles move much more quickly, resulting in increased pressure if the gas is confined.
direct proportionality
The kinetic energy of particles is directly proportional to temperature. Higher temperature means higher kinetic energy and more particle motion.
phase changes
In solids, increased temperature can lead to melting. In liquids, it can cause boiling. In gases, it results in increased pressure and volume (if not confined).
behaviour of matter
The increased motion of particles with temperature explains phenomena such as expansion, changes in state, and gas behavior under various thermal conditions.
the pressure of a gas
the forces exerted by particles colliding with surfaces, creating a force per unit area
What is the effect on the pressure of a fixed mass of gas of a change of temperature at constant volume?
Increase in Temperature: Particles gain more kinetic energy, move faster, and collide more frequently and forcefully with the container walls, resulting in increased pressure.
Decrease in Temperature: Particles lose kinetic energy, move slower, and collide less frequently and forcefully with the container walls, resulting in decreased pressure.
What is the effect on the pressure of a fixed mass of gas of a change of volume at constant temperature?
Decrease in Volume: Particles are compressed into a smaller space, leading to more frequent collisions with the container walls, resulting in increased pressure.
Increase in Volume: Particles have more space to move, leading to less frequent collisions with the container walls, resulting in decreased pressure.
What happens during the thermal expansion of solids at constant pressure?
Particles: Tightly packed, vibrating around fixed positions.
Expansion: Slight increase in volume due to increased vibration.
Degree of Expansion: Least among solids, liquids, and gases.
What happens during the thermal expansion of liquids at constant pressure?
Particles: Close together, able to slide past each other.
Expansion: Moderate increase in volume due to increased movement.
Degree of Expansion: More than solids, less than gases.
What happens during the thermal expansion of gases at constant pressure?
Particles: Far apart, moving freely.
Expansion: Significant increase in volume due to increased speed and spreading.
Degree of Expansion: Greatest among solids, liquids, and gases.
some of the everyday applications and consequences of thermal expansion
Thermal expansion affects everyday applications like thermometers and construction joints, while its consequences include thermal stress, dimensional changes in manufacturing, and impacts on railway safety or consumer electronics.
Know the melting and boiling temperatures for water at standard atmospheric pressure (limited to Celsius only)
At standard atmospheric pressure, the melting temperature of water is 0°C, and the boiling temperature is 100°C.
Describe the differences between boiling and evaporation
Boiling occurs at a specific temperature throughout the liquid with bubble formation, while evaporation occurs only at the surface at any temperature below the boiling point.
What happens to particles during condensation?
During condensation, gas particles lose energy, slow down, and come closer together to form a liquid.
What happens to particles during solidification (freezing)?
During solidification, liquid particles lose energy, slow down, and arrange into a fixed, orderly structure to form a solid.
How does temperature affect evaporation?
Higher temperatures increase evaporation by giving particles more energy to escape from the liquid surface.
How does surface area affect evaporation?
A larger surface area increases evaporation by exposing more liquid particles to the air.
How does air movement over a surface affect evaporation?
Increased air movement (wind) speeds up evaporation by removing the saturated air layer above the liquid, allowing more particles to escape.
evaporation
the escape of the more energetic particles from the surface of a liquid
evaporation causes
cooling of a liquid
Describe melting in terms of energy input without a change in temperature.
During melting, energy input breaks the bonds between solid particles, changing them into a liquid without increasing temperature.
Describe boiling in terms of energy input without a change in temperature.
During boiling, energy input breaks the bonds between liquid particles, changing them into a gas without increasing temperature.
examples of typical good thermal conductors
metals like copper, aluminum, and silver
examples of typical bad thermal conductors (thermal insulators)
materials like wood, plastic, rubber, and fiberglass.
Describe thermal conduction in solids in terms of atomic or molecular lattice vibrations.
In solids, thermal conduction occurs as atoms or molecules vibrate and transfer energy to neighboring particles in the lattice structure.
Describe thermal conduction in solids in terms of the movement of delocalized (mobile) electrons in metallic conductors.
In metallic conductors, thermal conduction occurs as delocalized (mobile) electrons move and transfer energy rapidly through the material.
convection in liquids and gases.
the transfer of heat through the movement of fluid particles. Warmer, less dense fluid rises, while cooler, denser fluid sinks, creating a circulating flow pattern.
Explain convection in liquids and gases in terms of density changes.
Convection occurs because warmer fluids expand and become less dense, causing them to rise, while cooler fluids contract and become denser, causing them to sink. This density difference drives the circulation of fluid and transfers heat energy.
Describe the effect of surface color (black or white) and texture (dull or shiny) on thermal radiation emission, absorption, and reflection.
Darker colors like black emit and absorb more thermal radiation than lighter colors like white. Rough or dull surfaces absorb and emit more radiation compared to smooth or shiny surfaces, which reflect more radiation.
How is the temperature of the Earth affected by thermal radiation?
The Earth's temperature is influenced by the balance between the thermal radiation absorbed from the Sun and the thermal radiation emitted by the Earth's surface and atmosphere.
Describe experiments to distinguish between good and bad emitters of thermal radiation
Use identical objects of different materials, heated to the same temperature, and observe which cools down faster; the faster-cooling material is a better emitter.
Describe experiments to distinguish between good and bad absorbers of thermal radiation.
Use identical objects with different surface finishes (e.g., polished and rough) exposed to thermal radiation sources, and measure the temperature change; materials that absorb more radiation show a greater temperature increase.
basic everyday applications of conduction
cooking utensils (burns), central heating systems (heat loss in buildings), coffee mugs
basic everyday applications of convection
room heaters, hair dryers, ocean currents
basic everyday applications of radiation
sunlight, infrared heaters, thermal cameras