(b) radioactivity
1/15
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
structure of an atom (7.2)
the nucleus contains protons and neutrons and the shell contains electrons. 146C
atomic (proton) number (7.3)
the number of protons in the nucleus of an atom
mass (nucleon) number (7.3)
the number of protons + the number of neutrons in the nucleus of an atom
isotope (7.3)
two isotopes of the same element have the same number of protons but a different number of neutrons
alpha (α) particles (7.4 / 7.5 / 7.7)
alpha radiation is a type of ionising radiation, meaning it is emitted from unstable nuclei in a random process.
an alpha particle is a helium nucleus
two protons, two neutrons (42He)
this means that when emitted, the atomic number goes down by 2 and the mass number goes down by 4
it has a large mass and a strong positive charge
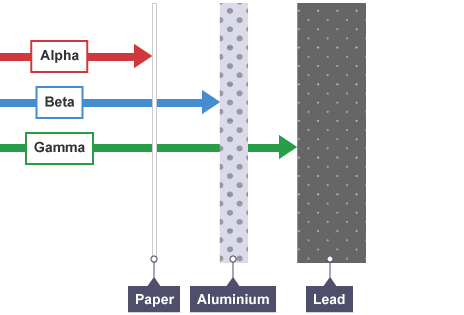
alpha radiation is the least penetrative and can be stopped by a sheet of paper, skin or a couple centimetres of air
beta (β-) particles (7.4 / 7.5 / 7.7)
beta radiation is a type of ionising radiation, meaning it is emitted from unstable nuclei in a random process.
a beta particle is a fast moving electron
this means that there is no change to the atomic or mass numbers when beta radiation is emitted
it has a very small mass and a negative charge
beta radiation is more penetrating than alpha radiation, so it can pass through skin, air and paper but is absorbed by a few centimetres of body tissue or a few millimetres of aluminium
gamma (γ) rays (7.4 / 7.5 / 7.7)
gamma radiation is a type of ionising radiation, meaning it is emitted from unstable nuclei in a random process.
gamma rays are high-energy electromagnetic waves caused by changes in nucleus
this means that there is no change to the atomic or mass numbers when gamma radiation is emitted
part of the electromagnetic spectrum (shortest wavelength) so it travels at the speed of light
there is no mass and no charge
gamma rays are very penetrative, easily penetrating body tissue and can only be stopped by a few centimetres of lead or a metre of concrete
investigate the penetration powers of different types of radiation using either radioactive sources or simulations (7.6)
do this with a geiger müller tube
ionising radiation detectors (7.9)
photographic film and geiger-müller detectors
sources of background (ionising) radiation (7.10)
radon gas from the ground, buildings and the ground, cosmic rays, food and drink, artificial sources (medical (x-rays, cancer treatment), nuclear power and weapons testing etc.)
the activity of a radioactive source …? (7.11)
decreases over a period of time and is measured in becquerels
half-life (7.12 / 7.13)
half life is time time it takes for half of the unstable nuclei in a sample to decay or for the activity of the sample to halve or for the count rate (number of decays recorded each second by a detector) to halve.
uses of radioactivity in industry and medicine (7.14)
x-rays and cancer treatment
contamination (7.15)
having radioactive atoms on something
irradiation (7.15)
exposure
dangers of ionising radiations (7.16)
mutations, can damage cells + tissue