Fielding Ch 19, 22, 23, 24; Glycocalyx MDRS; Lactate MDRs; Colloids SOTAs; IVF MDRs
1/437
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
438 Terms
Rhabdomyolysis
Skeletal muscle cell damage and necrosis resulting in the release of toxic intracellular contents from myocytes into the systemic circulation
Categories of Non-Exertional Rhabdomyolysis
Nutritional
Metabolic
Toxic
Traumatic (direct)
Infectious
Immune-mediated
Anesthesia-associated
Atypical myopathy
Non-Exertional Rhabdomyolysis - Nutritional
Selenium/vitamin E deficiency
Non-Exertional Rhabdomyolysis - Metabolic
Polysaccharide storage myopathy
Glycogen branching enzyme deficiency
Mitochondrial myopathy
Non-Exertional Rhabdomyolysis - Toxic
Ionophore toxicity
White snake root toxicity
Ivermectin
Cantharidin (blister beetle)
Non-Exertional Rhabdomyolysis - Infectious
Viral (equine influenz virus, equine infectiuos anemia, equine herpesvirus 1)
Bacterial (Clostridium spp)
Parasitic (Sarcocystis spp)
Non-Exertional Rhabdomyolysis - Immune-Mediated
Streptococcus-associated myositis
Non-Exertional Rhabdomyolysis - Anesthesia-Associated
Malignant hyperthermia
Compartment myopathy
Categories of Exertional Rhabdomyolysis
Sporadic
Chronic
Traumatic
Exertional Rhabdomyolysis - Sporadic
Inadequate fitness/training
Overexertion
Metabolic exhaustion
Heat stress
Exertional Rhabdomyolysis - Chronic
Recurrent exertional rhabdomyolysis
Polysaccharide storage myopathy
Dietary imbalance
Idiopathic
Exertional Rhabdomyolysis - Traumatic
Excessive struggling/cast
Genetic Abnormalities of Muscle Metabolism
Glycogen branching enzyme 1 deficiency in Quarter Horse and Paint foals
Genetic abnormality in the glycogen synthase 1 (GYS1) gene responsible for some horses with PSSM
Mutation in the RyR1 gene causing malignant hyperthermia in Quarter Horses
Atypical Myopathy (Seasonal Pasture Myopathy)
Severe and frequently fatal myopathy associated with ingestion of hypoglycin A within the seeds of the box elder tree and the sycamore maple
Magnitude in Elevation in CK in Proportion to the Degree of Muscle Damage and Severity of Clinical Signs
Magnitude of elevation in the muscle-specific enzyme CK is proportional to the degree of muscle cell necrosis, but not always reflected in the severity of the clinical signs
General Approach to Management of Acute, Severe Rhabdomyolysis
Minimizing further muscle damage by ceasing to move or exercise the horse
Replacement of intravascular volume and diuresis to prevent renal injury
Correction of acid-base and electrolyte abnormalities
Managing pain and anxiety
Pathophysiology of Acute Rhabdomyolysis
An increase in free ionized calcium in muscle cell cytoplasm is central to the pathogenesis of rhabdomyolysis
Normally, in resting muscle the sarcoplasmic Ca2+ concentration is strictly maintained within a very low range compared to that of the extracellular fluid
Allows a controlled increase in Ca2+ necessary for actin-myosin binding during muscle contraction
Any cause of persistently increased sarcoplasmic Ca2+ results in activation of destructive proteolytic enzymes and causes muscle cell membrane lysis, increased permeability, and eventual dissolution of the myocyte
Extensive muscle cell injury is accompanied by massive influx of sodium, chloride, and calcium into the cytoplasm, followed by massive efflux of potassium phosphate, myoglobin, cytosolic enzymes, and organic acids into the systemic circulation
Mechanisms of Increased Cytosolic Ca2+
Direct injury to the myocyte
Depletion of ATP which results in pump failure and a persistent increase in the sarcoplasmic Ca2+
`
Myoglobin-Induced Acute Kidney Injury
Myoglobin is released from damaged muscle cells into circulation and freely filtered by the renal glomeruli, where it is metabolized by tubular epithelial cells
Myoglobinuria only occurs as a consequence of rhabdomyolysis
Myoglobin is rapidly eliminated from plasma through renal clearance and has a short half-life (2-3 h in humans)
What are the three main mechanisms by which rhabdomyolysis is believed to lead to the development of AKI?
Renal vasoconstriction
Direct and ischemic tubule injury
Tubule obstruction by myoglobin cast formation
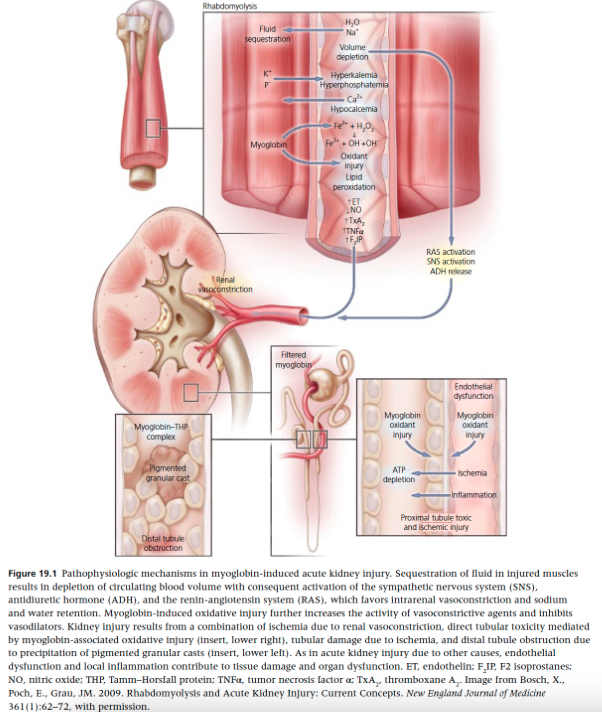
Mechanisms of Rhabdomyolysis that Lead to AKI - Renal Vasoconstriction
Intravascular volume depletion due to fluid accumulation within damaged muscle tissues activates RAAS, vasopressin, and sympathetic nervous system
Renal blood flow is also reduced by activation of vascular mediators including endothelin-1, thromboxane A2, TNF, and isoprostanes as well as a reduction in vasodilation by the scavenging of nitric oxide by myoglobin
Mechanisms of Rhabdomyolysis that Lead to AKI - Tubule Obstruction by Myoglobin Casts
Occurs principally in the distal tubules
Myoglobin is concentrated along the renal tubules, particularly in the face of volume depletion and renal vasoconstriction
Myoglobin cast formation occurs when myoglobin interacts with Tamm-Horsfall protein from damaged renal tubular cells, which is enhanced in acidic urine
Mechanisms of Rhabdomyolysis that Lead to AKI - Injury to Tubular Cells
Myoglobin contains iron in the form of ferrous oxide (Fe2+)
ROS are generated by the oxidation of ferrous to ferric oxide (Fe3+) which is favored in acidic urine
Oxidant injury and lipid peroxidation of proximal tubule cells results in direct tubule injury in the form of acute tubular necrosis
How to Determine the Presence of Myoglobinuria in Horses
Gross red-brown discoloration of urine
Urine dipstick test that is positive for blood in the absence of erythrocytes in the urine supernatant after centrifugation
Cannot distinguish myoglobin from hemoglobin
What is the most common acid base and electrolyte abnormality in horses with acute rhabdomyolysis?
Mild hypochloremic metabolic alkalosis
What are the typical electrolyte change reported to occur initially in acute rhabdomyolysis?
Hyperkalemia
Hyperphosphatemia
Hyperuricemia
Hypocalcemia
Hyperkalemia with Acute Rhabdomyolysis
Early manifestation of rhabdomyolysis
Can be a life-threatening complication
Further exacerbated by the presence of metabolic acidosis and development of renal failure
Hypocalcemia that also occurs in the early stages of rhabdomyolysis contributes to the cardiotoxic effects of hyperkalemia
Potassium concentrations generally peak 12-36 hours following muscle injury
Risk of life-threatening cardiac arrhythmias increases when potassium concentrations exceed 6.0 mmol/L and associated ECG changes are observed
Significant hyperkalemia is more likely to occur in severe cases of acute rhabdomyolysis and is less likely in horses suffering from rhabdo associated with prolonged endurance exercise
Hypocalcemia in Acute Rhabdomyolysis
Can occur in horses with severe rhabdomyolysis, particularly associated with exhausted horse syndrome
Initial hypocalcemia is due to the movement of calcium into damaged muscle cells and from precipitation of calcium and phosphate in necrotic muscle
Initial hypocalcemia is generally followed by gradually developing hypercalcemia as the patient is hydrated and developing hypercalcemia as the patient is hydrated and deposited calcium is mobilized from damaged muscle back into the circulation
In horses, may manifest as synchronous diaphragmatic flutter, which requires treatment that should include supplemental intravenous calcium
Metabolic Acidosis with Acute Rhabdomyolysis
Most common acid-base abnormality in humans with acute, severe rhabdomyolysis
Due to the release of organic acids, uric acid, phosphate, sulfate, and potassium from damaged muscle cells into the systemic circulation
Exacerbated by coexisiting hyperlactatemia due to hypovolemia and reduced tissue perfusion
Not a common feature in horses with acute rhabdo
Metabolic Alkalosis with Acute Rhabdomyolysis
Mild hypochloremic metabolic alkalosis is the most commonly reported abnormality in adult horses with rhabdo
Hypochloremia is proposed to occur as a result of excessive or prolonged sweating often associated with severe acute rhabdo
What is the most important intervention in preventing complications of rhabdomyolysis?
Early and aggressive IV fluid therapy represents the single most important intervention in preventing the development of complications of rhabdomyolysis
Fluid Replacement in Rhabdomyolysis
Optimal crystalloid fluid choice for resuscitation in horses with rhabdo is unknown but should be ideally be based on acid-base and electrolyte measurements performed on a blood sample collected at the time of presentation
When the acid-base and electrolyte status is unknown or normal, or if acidemia or aciduria is present, then large volumes of balanced isotonic replacement fluids such as LRS, Norm-R, Plasma-Lyte A, or Plasma-Lyte 148 would be appropriate
If focusing on small details, might consider LRS to be the fluid of choice with rhabdo because the acetate salt is primary metabolized by muscel tissue
Fluids should be delivered at high rates of 20-40 ml/kg/h until urination is observed and diuresis continued at 5-10 ml/kg/h until urine is clear, vital signs normalize, and serum creatinine is normal
In exhausted endurance horses with suspected rhabdomyolysis and in other cases in which a hypochloremic metabolic alkalosis is documented or suspected, the use of large volumes of isotonic (0.9%) saline for initial fluid replacement may be appropriate
Acid-base status should be re-evaluated following initial fluid replacement if possible
If hypochloremia and other electrolyte abnormalities have resolved, the continuing fluid therapy with a balanced isotonic crystalloid fluid would be recommended
Hypertonic Saline for Rhabdomyolysis
Significantly increases plasma volume expansion, as well as improving urine output in dehydrated endurance horses compared with isotonic (0.9%) saline alone
May have beneficial effects in preventing renal failure in horses with severe rhabdo
Judicious use of hypertonic saline in early resuscitation of horses with acute rhabdo showing signs of shock should be considered for its beneficial effects on plasma volume expansion, and improved tissue perfusion and renal blood flow
Dose should not exceed 4 ml/kg given as a bolus and then must be followed by additional fluid therapy with large volumes of balanced isotonic crystalloid fluids
Bicarbonate Therapy for Rhabdomyolysis
Indiscriminate use of bicarbonate therapy for urinary alkalinization in horses is not justified
Bicarbonate therapy is also not recommended in the initial fluid therapy management of horses with documented metabolic acidosis as most cases will resolve following adequate fluid volume replacement with IV isotonic crystalloid fluids
One exception is the treatment of severe, life-threatening hyperkalemia when glucose +/- insulin therapy fails to lower plasma K+ concentrations
If severe acidemia persists and/or urine pH is acidic despite adequate fluid replacement and diuresis, then bicarbonate therapy should be considered
Mannitol for Rhabdomyolysis
Osmotic effects of mannitol cause diuresis and increased urine output, as well as the redistribution of fluid from injured muscle tissue into the extracellular fluid space resulting in a reduction in tissue edema
Clinical studies have failed to show significant benefit of mannitol over aggressive large-volume crystalloid fluid resuscitation alone
Judicious use of mannitol and/or loop diuretics in the management of persistent anuria/oliguria in horses despite appropriate fluid therapy could be considered in the same manner as recommended for acute renal failure due to other causes
If forced diuresis with mannitol is employed in the management of acute rhabdomyolysis it must not be administered until adequate fluid replacement has been achieved
Correction of Hyperkalemia in Rhabdomyolysis
If the potassium concentration exceeds 6.0 mmol/L and/or ECG abnormalities are detected then potassium-lowering therapies should be continued using isotonic 0.9% saline combined with standard therapies that result in the movement of potassium from the ECF to the ICF (IV glucose, insulin, and/or sodium bicarbonate)
If unsuccessful, further therapy is aimed at enhancing potassium elimination through the use of diuretic agents, non-exchange resins, or dialysis
IV calcium has no direct effect on serum potassium concentrations but it is used to minimize the cardiotoxic effects of hyperkalemia by increasing the cell membrane threshold potential, thereby reducing membrane excitability
IV calcium is not recommended in the treatment of acute rhabdo so its use should be reserved for the treatment of hyperkalemia associated with life-threatening ECG changes
Intravenous Calcium Administration for Rhabdomyolysis
The decrease in extracellular calcium observed commonly in acute rhabdo is due to influx into the damaged muscle cells where it contributes to further cell damage and death
Administration of additional exogenous calcium is only likely to compound cell damage, and may contribute to soft tissue calcification
Calcium supplementation is not recommended as part of fluid therapy management unless life-threatening hyperkalemia or symptomatic hypocalcemia (tetany, synchronous diaphragmatic flutter) is observed
Dialysis for Rhabdomyolysis
Renal replacement therapy is recommended in human patients with refractory hyperkalemia, metabolic acidosis, or fluid volume overload associated with oliguric renal failure when other therapies have bee unsuccessful
Conventional hemodialysis does not remove myoglobin effective due to the large molecular size so intermittent hemodialysis is usually required
Peritoneal dialysis has been used effectively as a viable therapeutic alternative to hemodialysis in the management of horses with acute renal failure refractory to conventional therapies
Continuous peritoneal dialysis has been reported to be effective in the management of myoglobin-induced acute renal failure in an adult horse
Suggest that peritoneal dialysis be considered early in the management of horses with acute renal failure associated with rhabdo that does not respond adequately to conventional therapies
What % of body weight is the body water of foals in the first week of life?
Body water content of 71-83% of body weight during the first week of life compared to 60-70% in adult horses
ECF of Foals Compared to Adults
ECF compartment is larger in foals than adults
ICF of Foals Compared to Adults
Smaller in foals than adults
What % of total body water is the ECFV in foals?
ECFV represents approximately 49-50% of total body water in young foals as compared to 32% in the adult
What do neonatal foals have a greater requirement than adults?
Healthy foals have higher plasma and blood volumes than adult horses
Greater body water content, along with a higher metabolic rate, growth, and increased insensible losses (higher surface area to body mass ratio), results in a greater water requirement for neonatal foals as compared to adult horses
What is normal urine output for foals?
6 ml/kg/h
Primary reason for high urine output in foals is a high water intake - healthy foals nurse between 15 and 30% of their body weight in milk per day
What is normal USG of foal’s urine?
Mare's milk is approximately 89% water so the USG of newborn foals (with the exception of the first 24 h post partum) is usually hyposthenuric (<1.008) and is reported to range from 1.001 to 1.027 at 2 days of age
Foal’s USG at 0-6 Hours of Age
1.025 (1.008-1.035)
Foal’s USG at 12 Hours of Age
1.025 (1.003-1.041)
Foal’s USG at 24 Hours of Age
1.007 (1.001-1.031)
Foal’s USG at 48 Hours to 42 Days
Average 1.004-1.007 (range 1.000-1.016)
Foal’s USG at 58 Days of Age
1.010 (1.001-1.038)
Why is neonatal faol urine more concentrated during the first 12-24 hours of life?
Neonatal foal urine is more concentrated during the first 12-24 hours of life because of the presence of fetal urine and proteinuria (peak excretion at 6-12h post partum)
Proteinuria occurs due to filtration of small, low-molecular-weight milk and colostral protein fragments
When does the first urinary occur in healthy foals?
The first urination in healthy foals occurs at 6 and 10 hours for colts and fillies, respectively and healthy foals urinate every 1-2 hours during the first 2 weeks of life
Clinical Signs of Shock in the Foal
Abnormalities in the clinical perfusion parameters: obtundation, poor pulse quality, cool to cold extremities, prolonged capillary refill time, pale mucous membranes, poor jugular refill, and reduce urine output
Heart rate is variable in the hypovolemic foal and may not be reflective of hypovolemia so is an unreliable indicator
Causes of Shock in the Neonatal Foal
Hypovolemia among the most common
Septic shock
SIRS shock
Is dehydration or hypovolemia more difficult to detect in the neonatal foal?
Dehydration is more difficult to detect than hypovolemia in the neonatal foal
Dehydration - extravascular fluid deficits
Hypovolemia - intravascular losses
Laboratory Evidence of Shock in Foals
Hyperlactatemia (and sometimes lactic acidosis)
Increased oxygen extraction ratio
Blood or plasma lactate concentration
Foals commonly have increased lactate due to hypoperfusion, but also caused by SIRS, sepsis, and mitochondrial dysfunction
Blood lactate concentrations are highest at birth and decrease over time
Hyperlactatemia
Persistent, mild to moderate increases (2-5 mmol/L) in blood lactate concentration without a concurrent metabolic acidosis
Lactic Acidosis
Persistent moderate to marked increses in blood lactate concentration (usually >5 mmol/L) in association with metabolic acidosis
Arterial Blood Pressure as a Monitoring Tool for Foals in Shock
General guideline of 65-120 mmHg can be regarded as the normal mean blood pressure range and values should be interpreted in light of perfusion parameters, blood lactate, urine output, and other indicators of perfusion status
Premature foals may have lower pressures than term foals, which may be ample for organ perfusion
Central Venous Pressure as a Monitoring Tool for Foals in Shock
There are limitations to CVP as a marker of intravascsular volume status, a subnormal CVP generally is a reliable indicator of the need for fluid loading
When a high normal CVP is reached, additional bolus fluid administration should not continue
How is CVP measured in foals?
CVP can be measured in neonatal foals through the use of a 20-30 cm catheter inserted through the jugular vein but which terminates in the cranial vena cava
Placement can be confirmed through the use of radioopaque catheters and thoracic radiographs
CVP can be measured with a water manometer or pressure transducer
Measure CVP at end expiration
If a pressure transducer is used, the CVP is the mean of the a wave at the end of expiration
A wave represents atrial contraction
What is a normal CVP in healthy neonatal foals?
Ranges from 2.8-12 cmH2O
At what CVP should fluid volume loading cease?
Once a CVP of 10-12 cmH2O is reached, fluid volume loading should cease
If hypoperfusion is still evident, then inotrope (e.g. dobutamine) and pressor (e.g. norepinephrine) infusions should be instituted
Urine Output as a Monitoring Tool for Foals in Shock
Urine production is a key goal and endpoint to the resuscitative phase of fluid therapy
Initiation of urine flow in a previously anuric or oliguric foal is a reasonable indicator of establishment of renal perfusion
Once a dilute urine is produced (USG <1.020-1.025) then fluid boluses can cease
Urine output is beset measured in critically ill foals through the use of urinary catheterization
What % of fluid input should urine output equal once maintenance fluid therapy has been initiated in foals?
Once maintenance fluid therapy is initiated, urine output should equal or exceed 50-60% of fluid input unless excessive GI or insensible losses are present
What are the four components of a fluid plan?
Type of fluid
Rate
Objectives or goals
Limits or potential complications
Type of Fluids for Resuscitation in the Hypovolemic Foal
Crystalloids, colloids, or a combination can be used for fluid resuscitation of the hypovolemic foal
Hypertonic solutions, primarily hypertonic saline, are not commonly used in foals because they may not be as tolerant of large sodium loads
Their kidneys physiologically are not designed to eliminate large sodium loads under normal circumstances, as mare's milk is very low in sodium
Acute and marked hypernatremia and hyperchloremia can develop which is of particular concern in foals with renal disease or CNS injury
Balanced isotonic (or near isotonic) crystalloids are likely most physiologic for foals during the resuscitation or replacement phase of fluid therapy
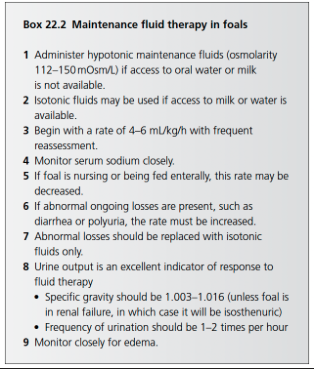
What should maintenance fluid therapy provide in foals?
Maintenance fluid therapy in foals is meant to maintain hydration, provide for abnormal ongoing losses such as diarrhea, or provide for diuresis as with azotemia
Fluids must sustain hydration in both the ECF and ICF
Free water is a necessity to ensure hydration of the ICF through osmolarity induced distribution
When can you use isotonic fluids for maintenance fluids in foals?
Isotonic fluids can be used when foals have an alternate free water source - either nursing or being fed milk/milk replacer
May be appropriate to use when ongoing losses are rich in sodium or electrolytes such as with diarrhea or enteritis
When should you use hypotonic fluids for maintenance in foals?
In foals without access to oral milk, hypotonic fluids are often selected to prevent development of hypernatremia and interstitital edema, and allow for hydration of the ICF
Colloids for Resuscitation in Foals
Until further research of synthetic colloids documenting efficacy or safety is available in foals, crystalloids for fluid resuscitation in foals
Plasma is the preferred colloid until further research is available regarding synthetic colloids
Plasma is commonly used for failure of passive transfer and colloid support but is not conducive to bolus administration as a resuscitative fluid because it must be thawed slowly and may be associated with adverse effects, especially if administered rapidly
What % of horses and foals receiving plasma have adverse effects?
About 10% of horses and foals receiving plasma have adverse effects that include urticaria, tachycardia, tachypnea, pyrexia, pruritis, swollen eyes, colic, and pyrexia
Estimating Fluid Deficits Based on % Dehydration in Foals
5% dehydration is regarded as the earliest that clinical signs of dehydration can be detected
Severe hypovolemic shock is thought to occur at 10-12% dehydration, which is associated with marked clinical and laboratory derangements including significant tachycardia and hemoconcentration
Fluid Challenge Method of Determining Amount of Resuscitation Fluids to Give
Estimating fluid deficits based on % dehydration may not be highly accurate and potentially be associated with error so the "fluid challenge" method circumvents these estimation
Consists of the administration of sequential boluses of 6-18 ml/kg of replacement crystalloids over 30 minutes
In foals an isotonic crystalloid dose of 10-20 ml/kg over 20-60 min
Bolus administration of colloids, at a smaller dose of 3-10 ml/kg may be used in addition to or instead of crystalloid fluids
If used, the colloid bolus should replace one or more of the crystalloid boluses in order to avoid hypervolemia
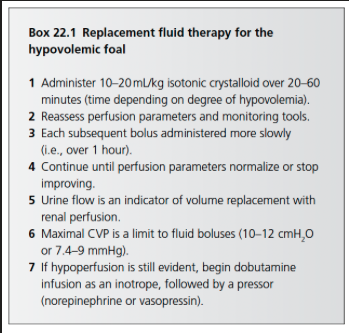
Serial Reassessment of Clinical Response to Fluid Challenges
Perfusion and hydration parameters should be evaluated at the complete of each fluid bolus to determine the need for further fluids
Perfusion: extremity temperature, mentation, pulse quality, CRT, and mucous membrane color
Production of urine is a positive sign that end organ perfusion has been established and rapid fluid boluses can be stopped or slowed
Hydration: skin turgor, corneal texture, and globe position
Most recumbent and hypovolemic foals require 2-3 of these 10-20 ml/kg boluses administered over the first several hours after presentation
A foal with persistent hypoperfusion, after having received 60-80 ml/kg of isotonic fluids, should be considered a candidate for inotrope therapy, followed by pressor therapy if blood pressure remains low
Objectives of Fluid Therapy in Foals
Replacement of lost fluids (plasma and interstitial volume for correction of hypovolemia and dehydration, respectively
Maintenance of hydration in a foal with water input that is below its requirements and losses
Diuresis for renal failure or urinary-excreted toxin removal
Replacement Fluid Therapy
Fluid resuscitation, administration of fluids to correct hypovolemia (loss of intravascular fluids) and dehydration (extravascular fluid loss)
What are the priorities of replacement fluid therapy in foals?
Priority of replacement fluid therapy is correction of hypovolemia (volume resuscitation) first, followed by addressing int
What are the goals of replacement fluid therapy in foals?
Resolve abnormal perfusion parameters: improved mentation, pulse quality, extremity temperature, mucous membrane color, CRT, and urine production
Urine production is best quantified with an indwelling urinary catheter, serial ultrasonographic examination of the bladder diameter during the resuscitative fluid phase is an effective means to monitor urine production if a urinary catheter is not in place
A bladder that increases in diameter during fluid therapy is a positive response
Improvement in arterial blood pressure
Response of Lactate Concentration to Fluid Therapy in Foals
Lactate concentrations may not normalize with fluid therapy in foals with SIRS states such as sepsis, even with re-establishment of perfusion
Causes of hyperlactatemia in foals includes hypoperfusion, inflammation (cytokines may inhibit pyruvate dehydrogenase activity), activation of the sympathetic nervous system (catecholamine surges), liver or kidney dysfunction, and mitochondrial dysfunction
Using Oxygen Extraction Ratio to Assess Tissue Perfusion
Oxygen extraction ratio (or venous oxygen saturation) can be used to assess tissue perfusion
With reduced oxygen delivery, the oxygen extraction ratio increases
Oxygen Extraction Ratio Formula
O2ER - SaO2-SvO2/SaO2
What is normal mixed venous oxygen tension in neonatal foals?
40.5 mmHg in recumbent foals
35.9 mmHg in standing foals
What is the goal of maintenance fluid therapy in foals?
Maintenance fluid therapy goal is to provide fluids necessary to maintain hydration status and fluid balance
Limits (Endpoints) of Resuscitation Phase of Fluid Therapy in Foals
Termination of the resuscitation phase of fluid therapy occurs when either goals are reached (urine flow, normalization of perfusion parameters, improvement in lactate, arterial blood pressure, and oxygen extraction ratios) or when limits are reached (maximum normal CVP, edema)
A negative CVP and values below 2 cmH2O are indicative of hypovolemia
CVP values within the normal range do not rule out hypovolemia because they can be increased by raised pleural pressure, cardiac dysfunction, and venous tone
Values above 12 cmH2O represent a clear indication to stop rapid fluid administration
A decrease in arterial oxygen saturation as measured with pulse oximetry or arterial blood gas analyses, as well as an increase in respiratory rate or character during fluid therapy also warrant decreases or discontinuation fluid boluses because they could be indicators of developing pulmonary edema
Basal Daily Water Requirement of the Neonatal Foal
Closer to 4-6 ml/kg/h rather than 10 ml/kg/h
Holliday-Segar Formula
Holliday-Segar formula
100 ml/kg/day for the first 10 kg of the foal's body weight
+50 ml/kg/day for the second 10 kg of the foal's body weight
+25 ml/kg per day for the remainder of the body weight
Relatively fluid restrictive rate or "dry maintenance rate"
Maintenance Fluid Therapy in Foals
Sodium Considerations with Fluid Therapy in Foals
Neonatal foals have a relatively low requirement for sodium as indicated by the low sodium content of mare's milk (<20 mEq/L and <15 mEq/L on days 0-2 and >3 days of lactation, respectively
A foal nursing 20% of its body weight in milk receives approximately 1.5-2.8 ml/kg/day of sodium
Administration of Norm-R evaluated as a maintenance fluid caused a rise in serum sodium and osmolarity as well as urine sodium and osmolarity and urine-to serum osmolarity ratio indicating that this fluid was not an ideal maintenance fluid for foals
In addition, decrease in urinary fractional excretion of potassium suggested that total body potassium stores decreased
Suggest that isotonic balanced electrolyte solutions, which are meant to be used as replacement fluids, are inappropriate for use as maintenance fluids in neonatal foals because of excessive sodium and inadequate provision of potassium
Correcting Hyponatremia in Foals
Hyponatremia should be corrected at a rate not exceeding 0.5 mEq/h to avoid central pontine myelinolysis associated with more rapid correction
If hyponatremia induced seizures are present, hyponatremia can be partially corrected by increasing the plasma sodium concentration by 2-4 mEq/L rapidly (not to exceed 125 mEq/L) in order to abolish seizure activity
Correcting Hypernatremia in Foals
Hypernatremia should be corrected at a similarly slow rate of 0.5 mEq/h to avoid development of cerebral edema
Acute hypernatremia can be corrected more rapidly
Potassium Considerations with Fluid Therapy in Foals
Mare's milk is relatively high in potassium
Sick foals that are not consuming milk have little to no potassium ingestion and require supplemental potassium in IV fluids
Supplementation is usually empiric with 10-40 mEq/L of potassium chloride supplemented
For foals with hyperchloremia, potassium phosphate can be used for a portion of the supplementation (up to 10 mEq/L)
Phosphorous can be safely supplemented at 0.01 mmol/kg/h
Maximum safe dose of potassium supplementation is 0.5 mEq/kg/h
Correcting Hyperkalemia in Foals
Hyperkalemia may be present in critically ill foals, especially those with uroperitoneum, acute renal failure, HYPP, severe rhabdo, and adrenal insufficiency
Can be treated with administration of 20 ml/kg of 0.9% NaCl containing calcium (0.4-1 m/kg of 23% CaGlu) and 4-8 mg/kg/min of dextrose with the fluid rate depending on the degree of hypovolemia
Can be followed by an additional 10-20 ml/kg of 0.9% sodium chloride containing 1 mEq/kg sodium bicarbonate and dextrose
Alternatively, isotonic sodium bicarbonate can be used with added dextrose
Don't mix sodium bicarbonate and calcium in the same bad
Insulin may be required in cases of severe or refractory hyperkalemia or when hyperglycemia is already present (starting with 0.005-0.01 unit/kg/h IV of regular insulin)
Cation exchange resins (sodium polystyrene sulfonate) can be administered as enemas or orally to absorb potassium
Calcium Considerations with Fluid Therapy in Foals
Significant ionized hypocalcemia is uncommon in neonatal foals except in cases of prolonged anorexia or those with hypoparathyroidism or parathyroid dysfunction
Mild hypocalcemia is common among septic or endotoxemic foals
Alkalosis or rapid administration of sodium bicarbonate can reduce the ionized calcium fraction by increasing binding of calcium to albumin
Calcium supplementation of fluids is usually empiric
Generally ionized calcium concentrations of 1.1-1.3 mmol/L are used as cut-offs to begin supplementation
Total volume of administered CaGlu should not exceed 1 ml/kg unless warranted by ionized calcium concentrations
Dextrose Considerations with Fluid Therapy in Foals
Foals that have not been nursing or do not tolerate an adequate level of enteral nutrition require dextrose supplementation
Parenteral nutrition consisting of dextrose, amino acids, and possibly lipids is indicated if neonatal foals continue to be intolerant of enteral feeding within 12-24 hours
Dextrose requirement of the late-term fetus is 4-8 mg/kg/min
During bolus fluid administration in foals with hypoglycemia or those that have been anorexic, fluids should contain only 0.6-1.2% dextrose if fluids are bolused at a rate of 20 ml/kg over 30 minutes to avoid hyperglycemia
Replacement Fluids for Foals
LRS
Plasmalyte A
Plasmalyte 148
Normosol R
Physiologic Saline
What is the difference between Plasmalyte A and Plasmalyte 148?
The in vitro pH
5.5 in Plasmalyte 148
7.4 in Plasmalyte A
Electrolyte Contents of Plasmalyte and Normosol vs LRS
The electrolyte contents of Plasmalyte and Normosol are more similar to normal equine plasma than LRS
Sodium-chloride difference in Plasmalyte and Normosol is 42 and its 21 in LRS
Approximately 40 in equine plasma
Plasmalyte and Normosol have a neutral to alkalinizing effect from an electrolyte content whereas LRS may be acidifying from an electrolyte standpoint but may be alkalinizing once the lactate is metabolized
Larger SID in Plasmalyte 148 or A which is preferred in patients with metabolic acidosis
What are the alkalinizing salts in Plasmalyte A/148 and Normosol R? How are they metabolized?
Acetate and gluconate
Acetate metabolized primarily by muscle tissue but also kidneys, liver, and other tissues
Gluconate metabolized by many tissues
Indicated in horses with liver dysfunction because lactate metabolism is reduced
What is the alkalinizing salt in LRS? Where is it metabolized?
Sodium lactate
Lactate metabolized by the liver where it ultimately undergoes gluconeogenesis or is oxidized to carbon dioxide and water