8. Carboxylic Acid
0.0(0)
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
Last updated 3:57 PM on 3/31/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
What are the properties of Carboxylic Acids?
has a carboxyl group (-COOH/carbonyl and hydroxyl)
weak acids (because of the presence of H+ ions)
very polar
2
New cards
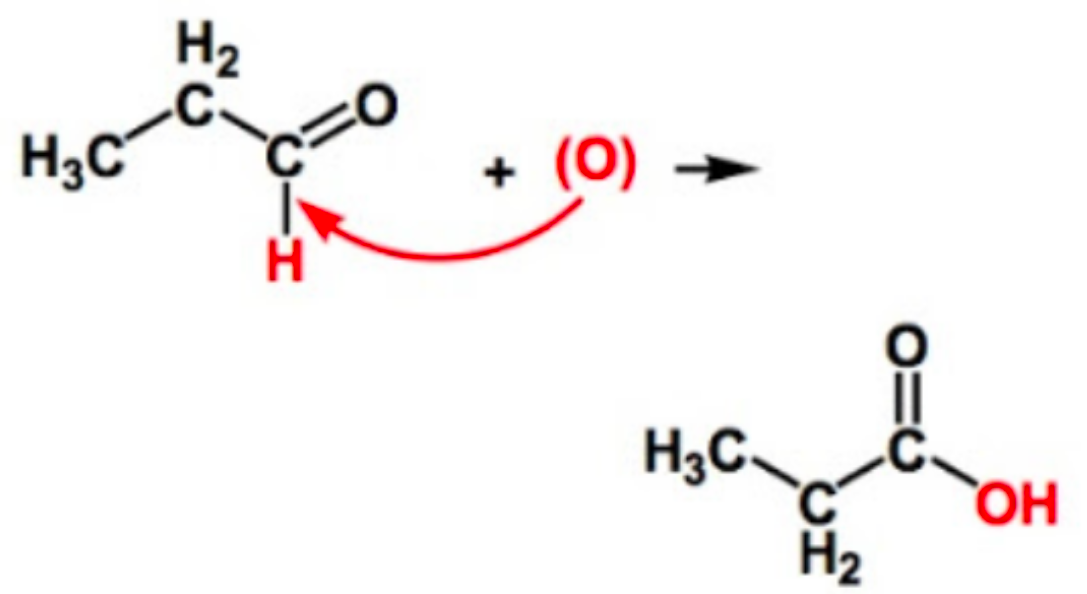
How are carboxylic acids formed?
controlled oxidation of aldehydes with oxidizing agents
3
New cards
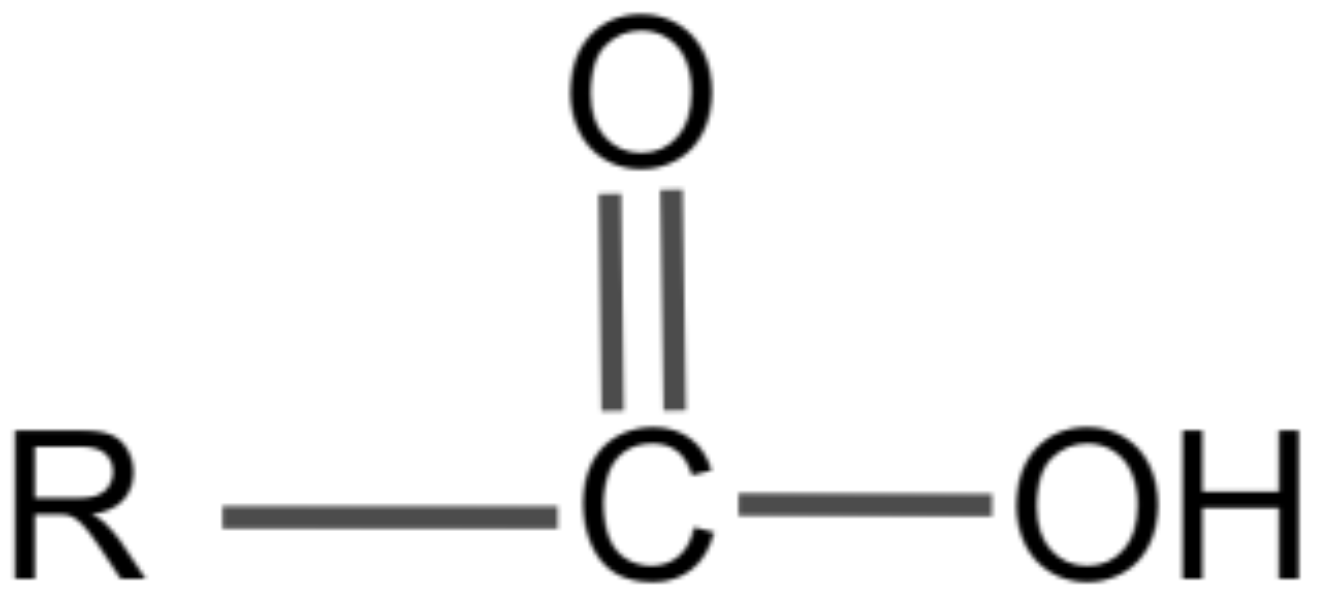
General Structure for Carboxylic Acids
R is a hydrocarbon
General formula: CₙH₂ₙO₂
4
New cards
How do you name Carboxylic Acids?
first part is the alkane
drop the -e and change it to -oic acid
no need for a locant because the carboxyl is always terminal carbon
5
New cards
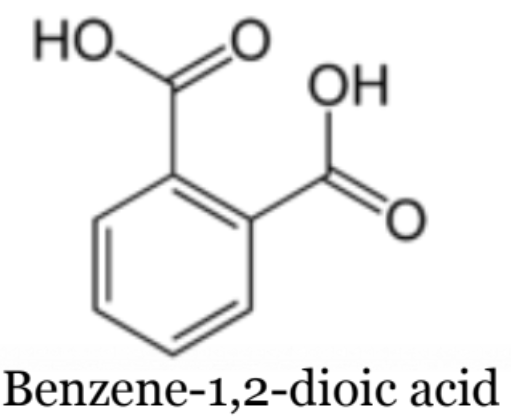
Dicarboxylic Acids
an acid with 2 carboxyl groups
when naming, it goes:
full name - location - dioic acid
6
New cards
Relationship between volatility and molecule
