CHEM 215-2 Exam 1
1/53
Earn XP
Description and Tags
organic synthesis, ch 18-21; some questions are kinda repeats with different context/wording
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
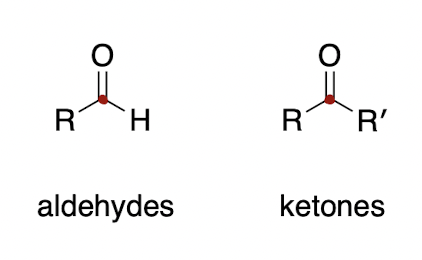
why are aldehydes (O=CH) more reactive than ketones (O=C)? → two reasons
1. aldehydes are less sterically hindered
2. ketones have 2 EDGs while aldehydes only have 1, so carbon in aldehyde is more delta positive and more likely to experience Nuc attack
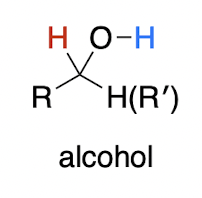
![<p><strong>metal hydride </strong>(irreversible) <br><br>aldehyde/ketone [2] → ?<br>1,2 or 1,4 addition?</p>](https://knowt-user-attachments.s3.amazonaws.com/fb5f5636-ef17-4b72-a4c2-9fcc6bcc788d.png)
metal hydride (irreversible)
aldehyde/ketone [2] → ?
1,2 or 1,4 addition?
alcohol with lone H
always 1,2-addition
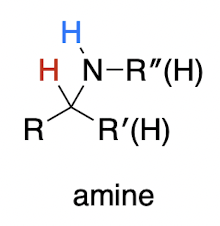
![<p><strong>metal hydride </strong>(irreversible)</p><p>imine ([2], N=C) → ?<br>1,2 or 1,4 addition?</p>](https://knowt-user-attachments.s3.amazonaws.com/b32aa8e2-f21c-44b3-9b90-d4a00835134c.png)
metal hydride (irreversible)
imine ([2], N=C) → ?
1,2 or 1,4 addition?
amine
always 1,2-addition
![<p><strong>metal hydride </strong>(irreversible) <br>* why does it only work with LiALH4</p><p>nitrile ([3], CN) → ?<br>1,2 or 1,4 addition?</p>](https://knowt-user-attachments.s3.amazonaws.com/a9c005fb-0123-4cdb-ac46-52db839a1854.png)
metal hydride (irreversible)
* why does it only work with LiALH4
nitrile ([3], CN) → ?
1,2 or 1,4 addition?
amine
always 1,2 addition
only works with LiAlH4 (hammer) because it’s more reactive, can reduce [3] to [1] → which is why it needs to be introduced in separate steps
![<p>amine<br>always 1,2 addition</p><p>only works with LiAlH4 (hammer) because it’s more reactive, can reduce [3] to [1] → which is why it needs to be introduced in separate steps</p>](https://knowt-user-attachments.s3.amazonaws.com/38d2151e-ac29-4734-b615-a00b4b4b00a8.png)
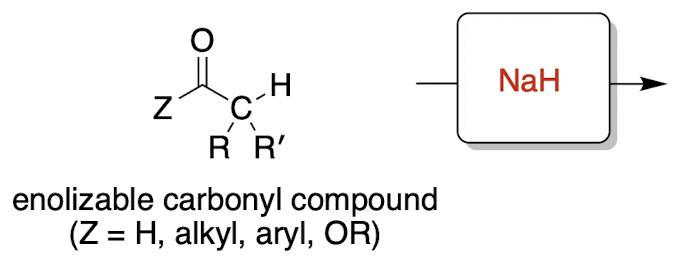
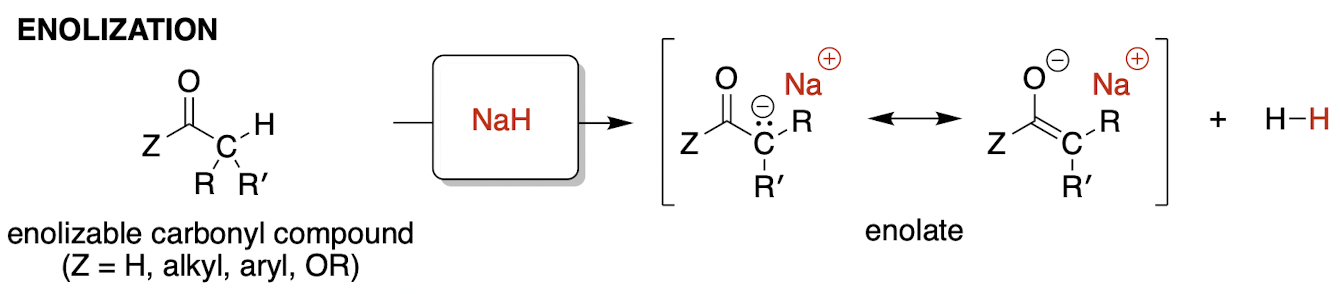
enolization (irreversible)
enolizable = at least one hydrogen on its alpha carbon
H2 ↑
![<p><strong>organometallic</strong>, e.g. Grignard (irreversible) </p><p>aldehyde/ketone [2] → ?</p>](https://knowt-user-attachments.s3.amazonaws.com/6e1f901e-4442-458d-b65b-2254c87aecb0.png)
organometallic, e.g. Grignard (irreversible)
aldehyde/ketone [2] → ?
alcohol with R group
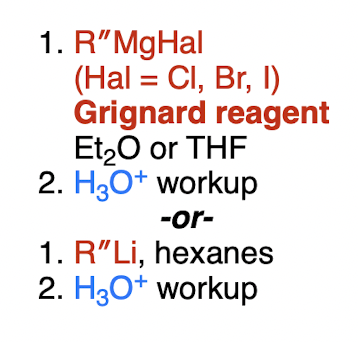
organometallic, e.g. Grignard (irreversible)
carbon dioxide (O=C=O) → ?
![<p><strong>organometallic</strong>, e.g. Grignard (irreversible) </p><p>nitrile ([3], CN) → ?</p>](https://knowt-user-attachments.s3.amazonaws.com/7f9e087e-3ed5-497a-9919-a4d3b6719174.png)
organometallic, e.g. Grignard (irreversible)
nitrile ([3], CN) → ?
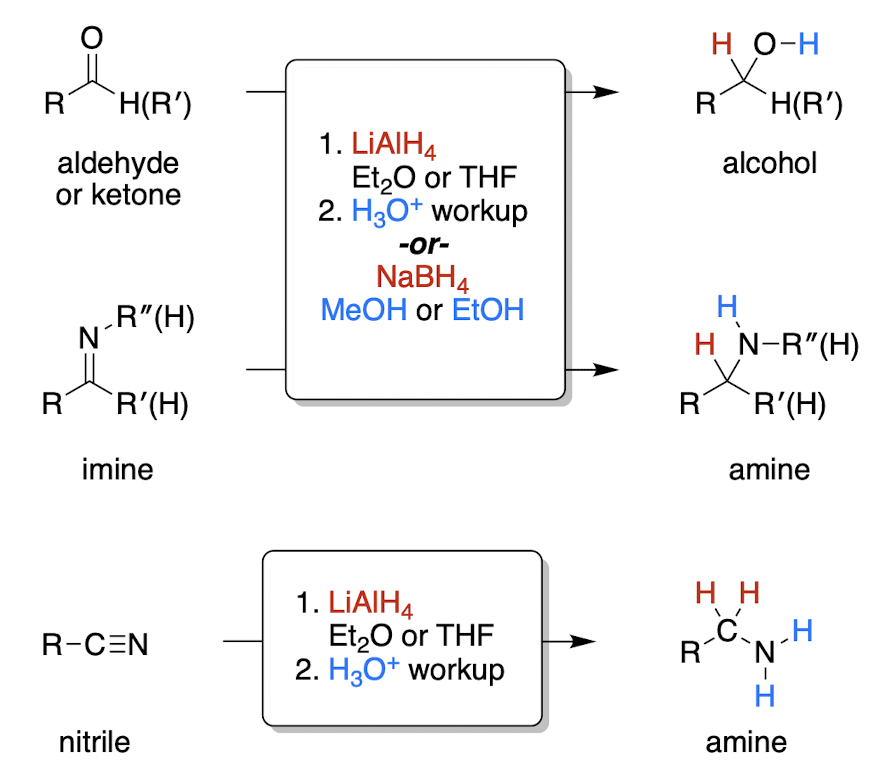
metal hydride mechanism
* what do LiAlH4 and NaBH4 act as?
LiAlH4 and NaBH4 = H- (strong base)
Nuc attack of H- on central carbon to reduce C=O bond, then protonation of O (acid workup)
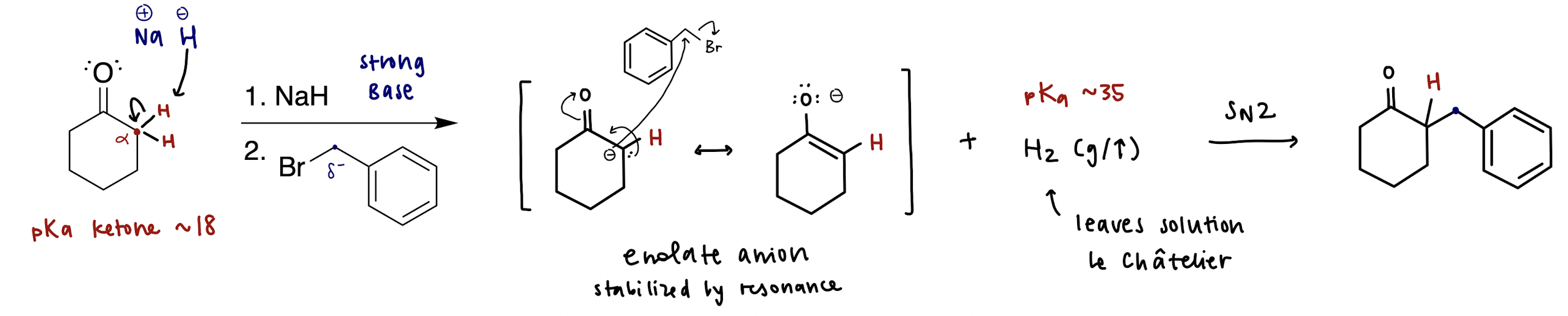
enolization mechanism
* enolate is a deprotonated carbonyl, which is defined by C=O
NaH = H- (non-nucleophilic, strong base)
H- deprotonates alpha carbon to form enolate through resonance, and hydrogen gas (H2) leaves the mixture
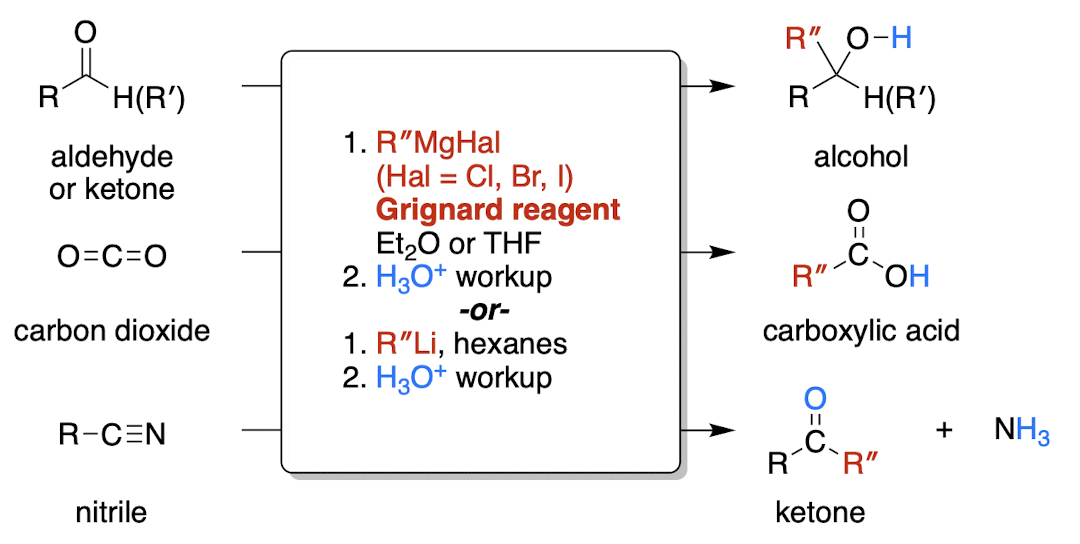
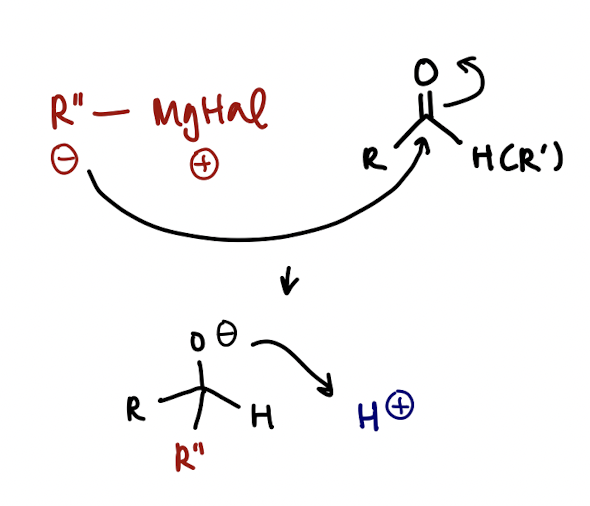
organometallic, e.g. Grignard mechanism
MgHal has a positive charge & is a spectator ion
Nuc attack of R- on central carbon to reduce C=O bond, then protonation of O (acid workup)
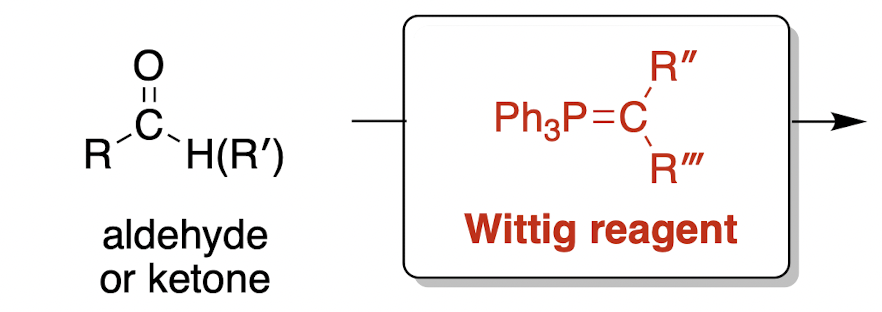
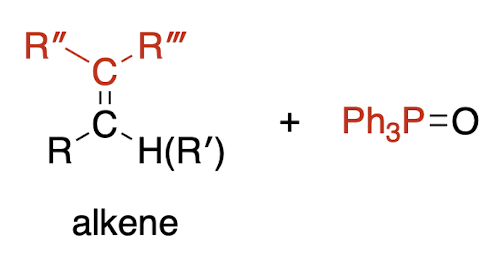
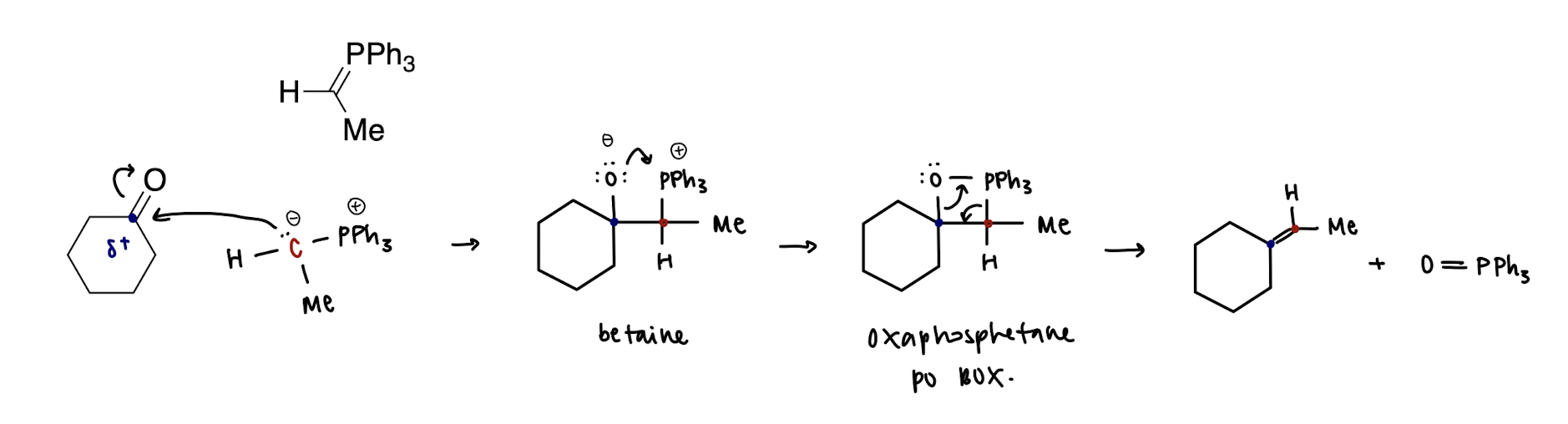
Wittig olefination; olefin = alkene (irreversible)
Wittig reagent is a phosphonium ylide, where a ylide is a compound with opposite charges on adjacent atoms where both atoms have an octet
organometallic reagents are incompatible with…
…acidic groups such as OH and NH
this is because Grignard and organolithium reagents are powerful bases that cannot be used as nucleophiles on compounds which contain acidic hydrogens
Wittig reaction mechanism
formation of the PO box!
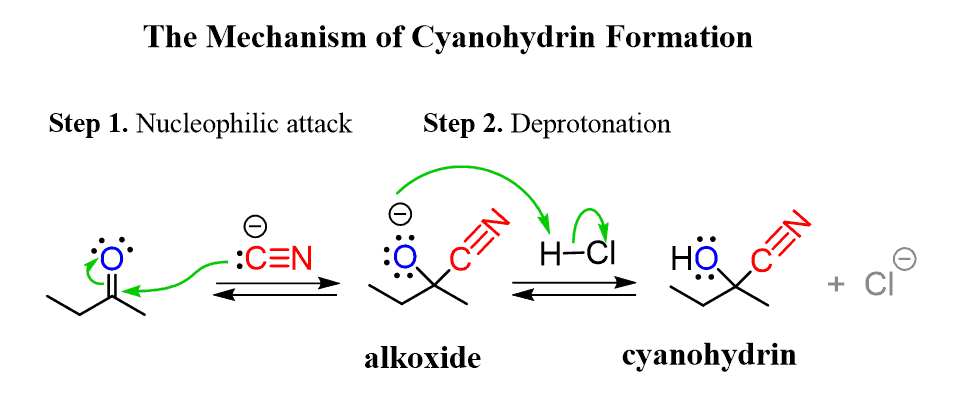
cyanohydrin formation (reversible)
aldehyde/ketone [2] → ?
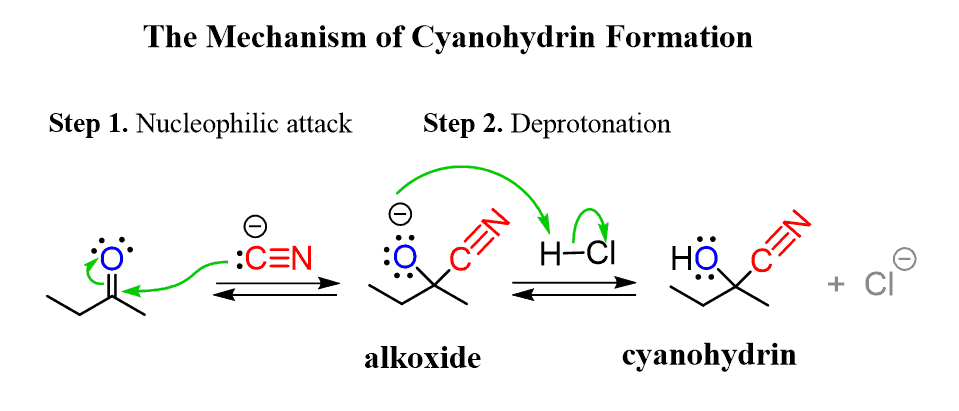
cyanohydrin formation mechanism
Nuc attack of CN- on central carbon to reduce C=O bond, then protonation of O (acid workup)
imine/Schiff base formation (reversible)
what are the
1. reagents
2. conditions
3. products?
aldehyde/ketone [2], RNH2 (1º amine)
H+ catalyst, buffered 4-5 pH
imine (C=N)
![<ol><li><p>aldehyde/ketone [2], RNH2 (1º amine)</p></li><li><p>H+ catalyst, buffered 4-5 pH</p></li><li><p>imine (C=N)</p></li></ol>](https://knowt-user-attachments.s3.amazonaws.com/5298c943-b46a-41f4-b7bf-1facee229494.png)
enamine formation (reversible)
note: first five steps are the same as imine formation
what are the
1. reagents
2. conditions
3. products?
aldehyde/ketone [2], R2NH (2º amine)
H+ catalyst, buffered 4-5 pH
imine (C=N)
![<ol><li><p>aldehyde/ketone [2], R2NH (2º amine)</p></li><li><p>H+ catalyst, buffered 4-5 pH</p></li><li><p>imine (C=N)</p></li></ol>](https://knowt-user-attachments.s3.amazonaws.com/979331b3-9c09-45db-bf17-e7adad149a79.png)
reductive amination (reversible)
this produces a new amine (e.g. 1º → 2º) through 2 steps
amine → imine through imine/Schiff base formation
reduction (e.g. with NaBH4 or LiALH4)
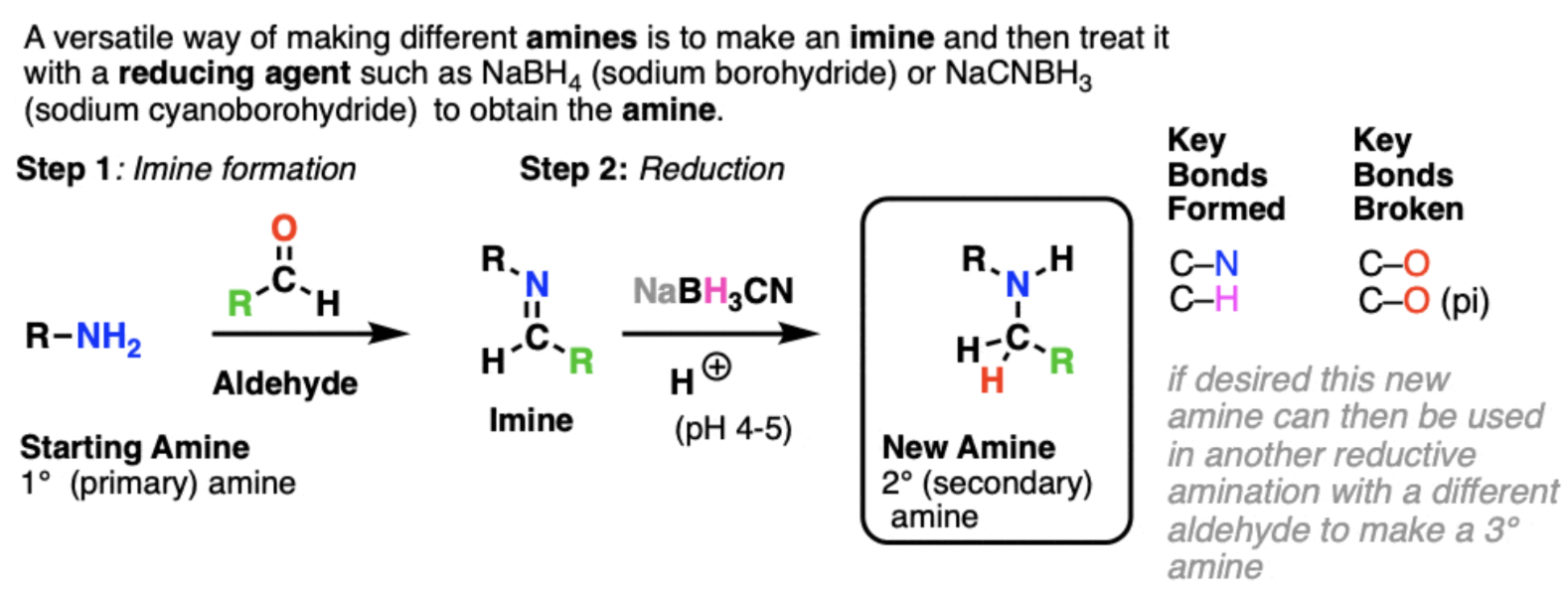
how can reductive amination occur in ONE STEP
with modified borohydride reducing agents
e.g. sodium cyanoborohydride, NaBH3CN, and sodium triacetoxyborohydride, NaBH(OAc)3
![<p><strong>Wolff-Kischner reaction </strong>(reversible)<br>* only with Class A carbonyl</p><p>aldehyde/ketone [2] → ?</p>](https://knowt-user-attachments.s3.amazonaws.com/03df95af-2edb-4960-91b1-c214f9c76d95.png)
Wolff-Kischner reaction (reversible)
* only with Class A carbonyl
aldehyde/ketone [2] → ?
C=O is completely deleted and replaced with two H, N2 ↑
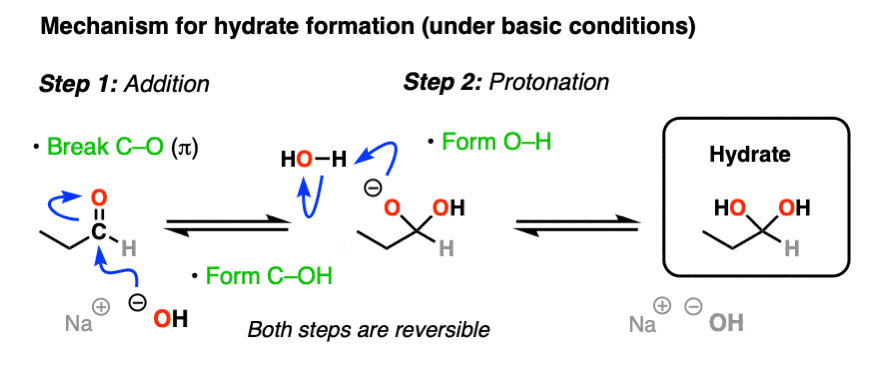
hydrate formation (reversible)
aldehyde/ketone [2] + ? → hydrate
what’s another name for hydrate?
H2O, H+ catalyst
forms a geminal diol
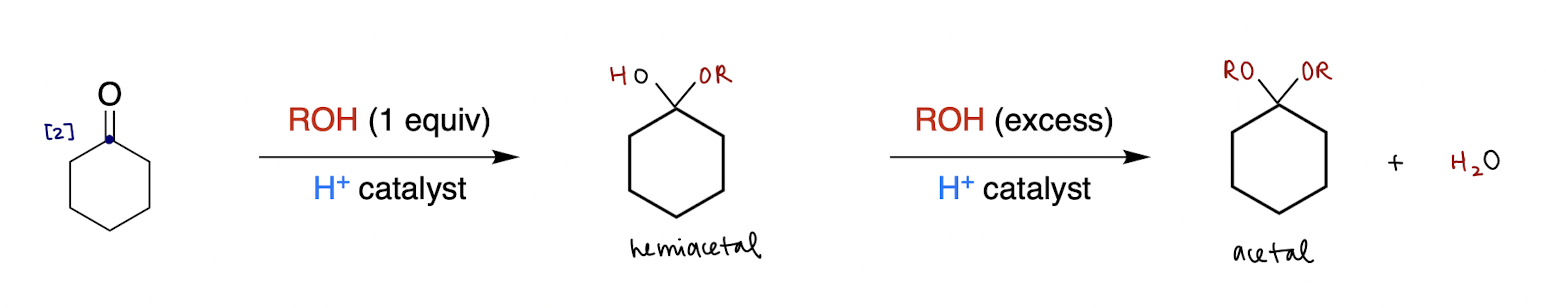
hemiacetal/acetal formation (reversible)
aldehyde/ketone [2] + ? → hemiacetal
““ + ? → acetal
ROH (1 equiv), H+ catalyst
ROH (excess), H+ catalyst
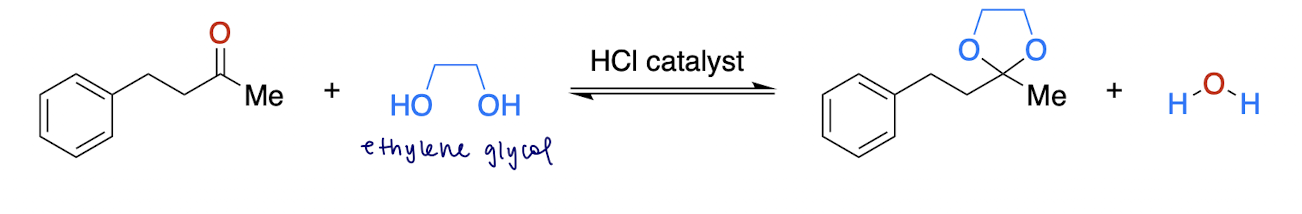
cyclic acyl formation (reversible)
aldehyde/ketone [2] + ? → cyclic acyl
glycol diol, H+ catalyst
![<p><strong>base-catalyzed nitrile hydrolysis </strong>(reversible)</p><p>nitrile (class B carbonyl or [3]) + H2O and ? → ? </p>](https://knowt-user-attachments.s3.amazonaws.com/a471af82-3e65-4d81-bc6c-34ddeb0a7146.png)
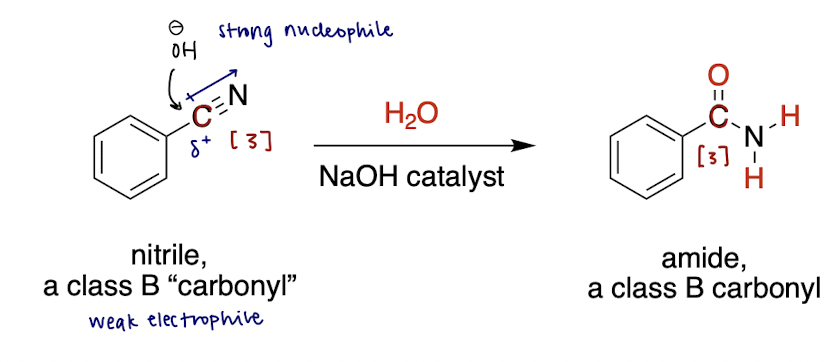
base-catalyzed nitrile hydrolysis (reversible)
nitrile (class B carbonyl or [3]) + H2O and ? → ?
H2O with base (e.g. NaOH) catalyst
OH Nuc attack the C in nitrile
forms an amide (class B, redox-neutral)
![<p><strong>acid-catalyzed nitrile hydrolysis </strong>(reversible)</p><p>nitrile (class B carbonyl or [3]) + H2O and ? → ? </p>](https://knowt-user-attachments.s3.amazonaws.com/1c42769c-22dc-45ab-a3d1-80189618b88b.png)
acid-catalyzed nitrile hydrolysis (reversible)
nitrile (class B carbonyl or [3]) + H2O and ? → ?
H2O with acid (e.g. H+) catalyst
H bonds to EN N in nitrile
forms an amide (class B, redox-neutral)
aldol reaction summary
enolate ion reacts with a class A carbonyl compound to form a β-hydroxycarbonyl
base catalyzed enol addition mechanism (reversible)
OH- deprotonates α-carbon to create enolate, carbanion in resonance structure bonds to class A carbon
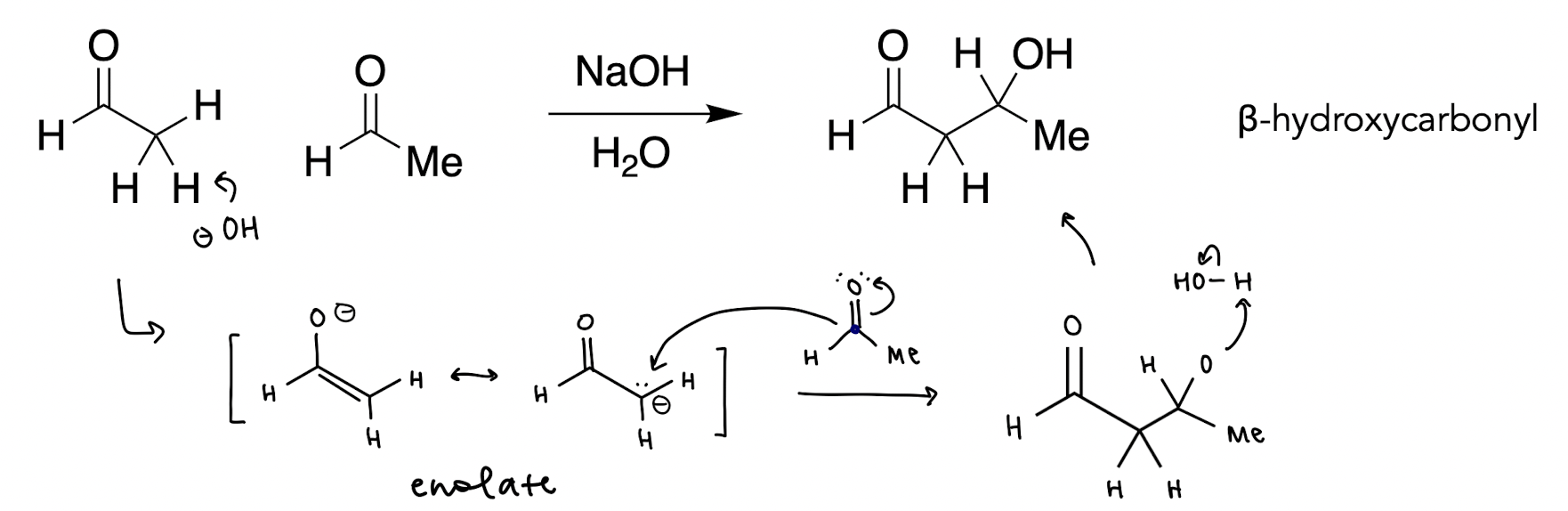
base catalyzed enol condensation mechanism (reversible)
* simple mechanism
OH- deprotonates α-carbon to make OH a decent leaving group
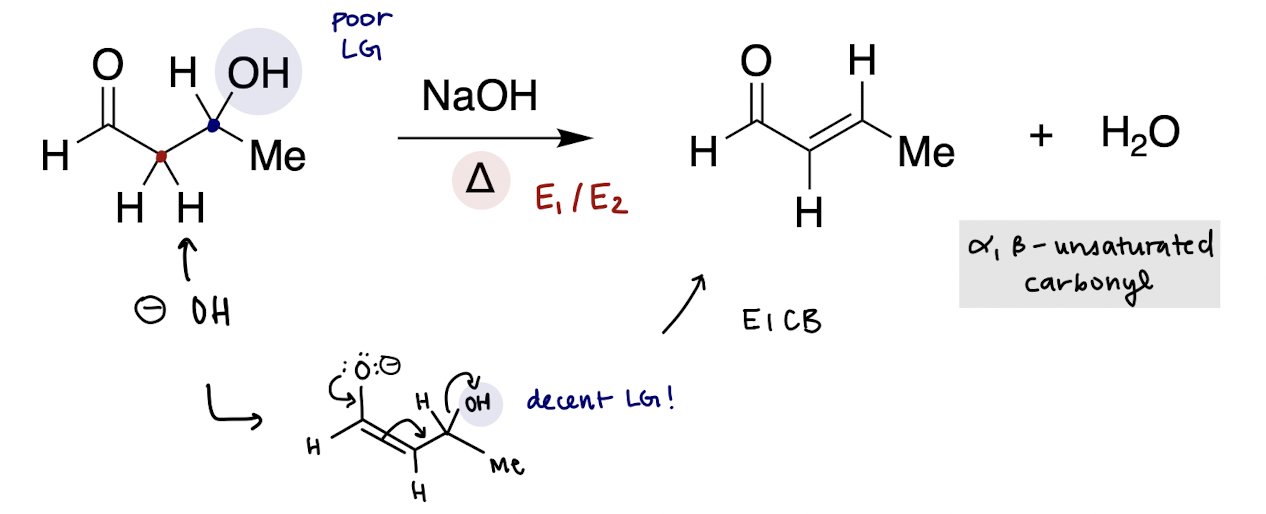
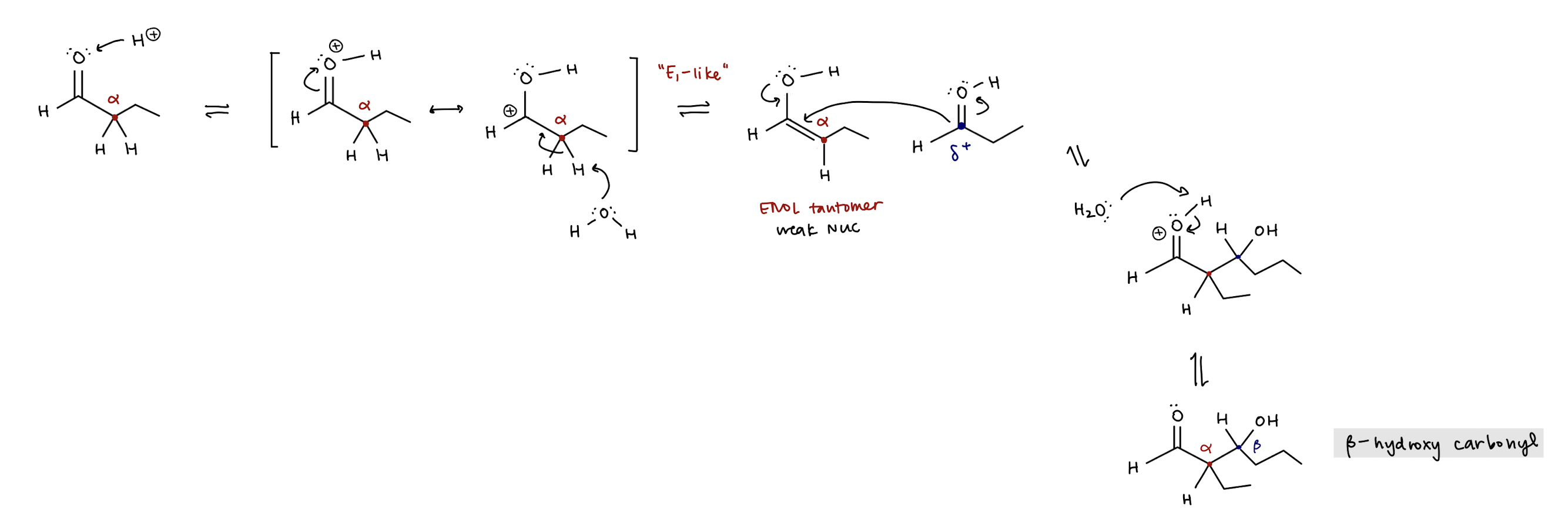
acid catalyzed enol addition mechanism (reversible)
acid (H+) protonates O, α-carbon is deprotonated by water to create alkene that bonds to class A carbonyl at β-carbon
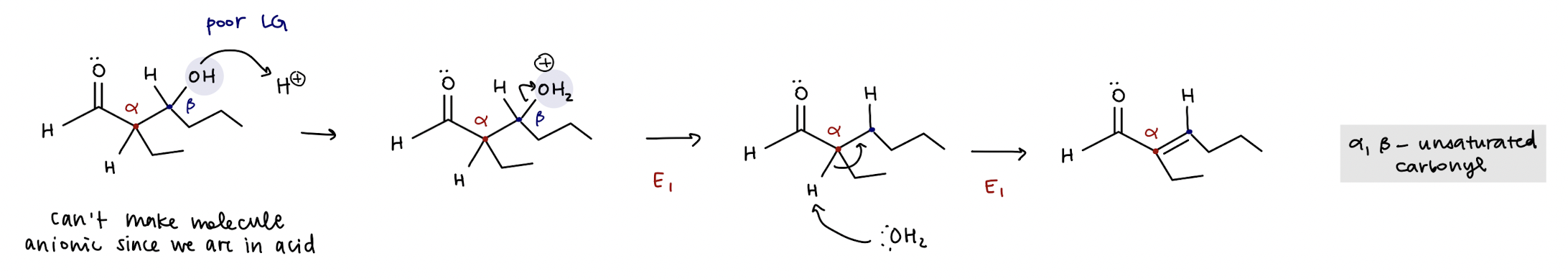
acid catalyzed enol condensation mechanism (reversible)
turn OH → H2O (good LG), α-carbon is deprotonated by water to create alkene
mnemonic for aldol reactions :D
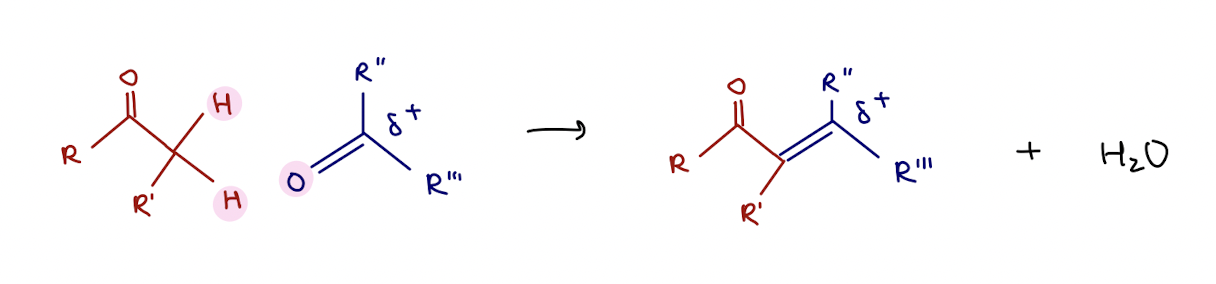
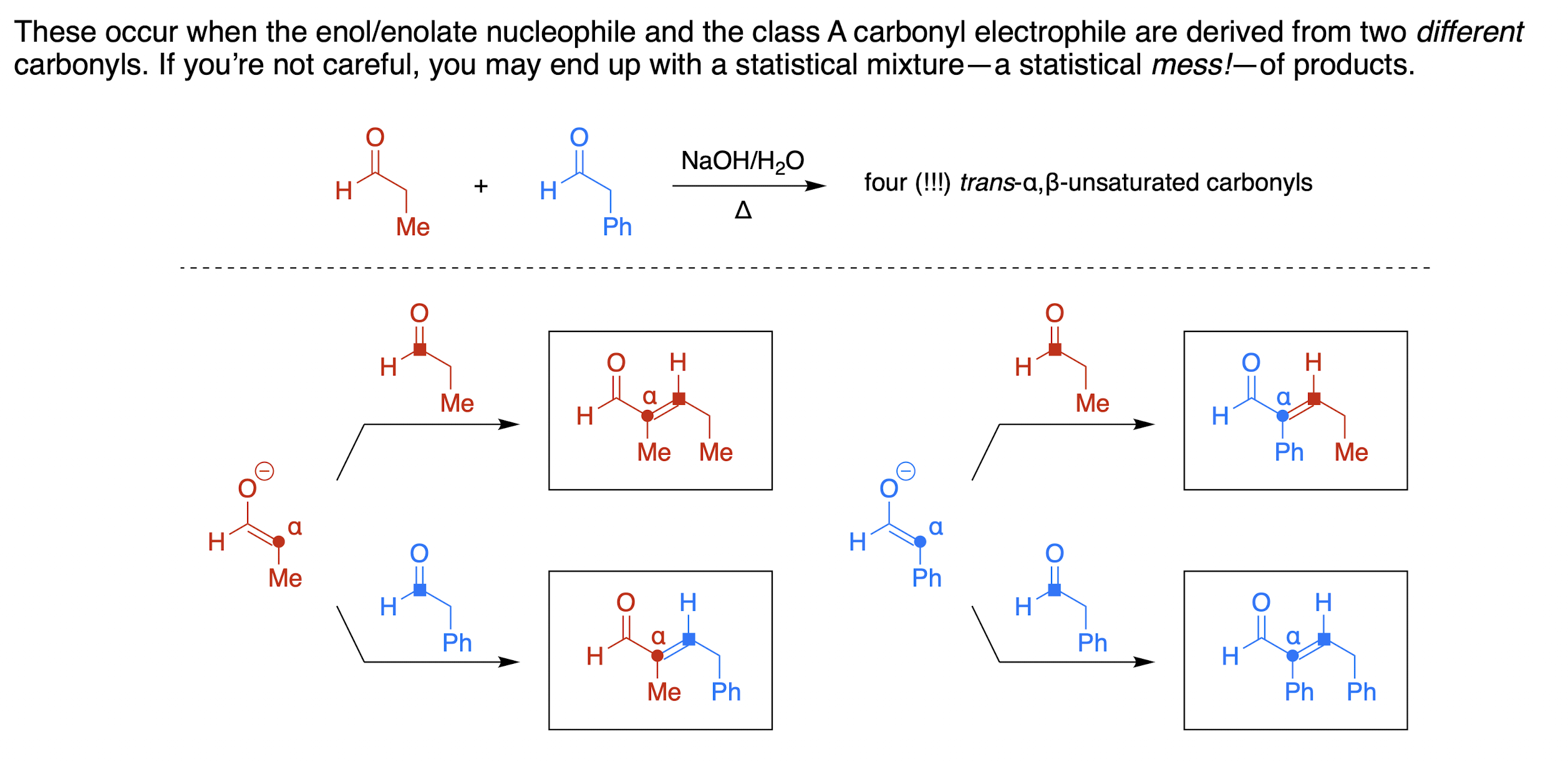
how to make a “crossed aldol reaction” useful?
choose an electrophile that lacks α-hydrogens (e.g. aryl aldehydes)
Directed Aldol Reaction: add a very strong base, especially useful if there are multiple enolizable protons
intramolecular aldol reaction
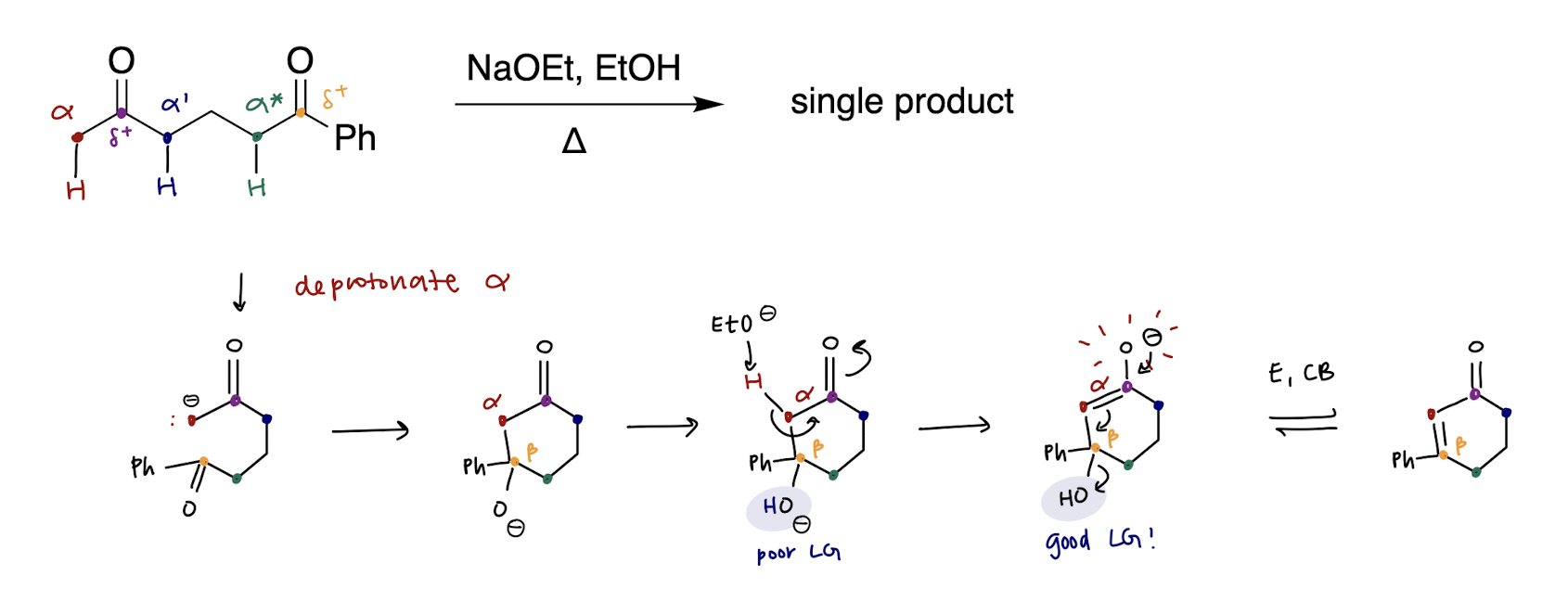
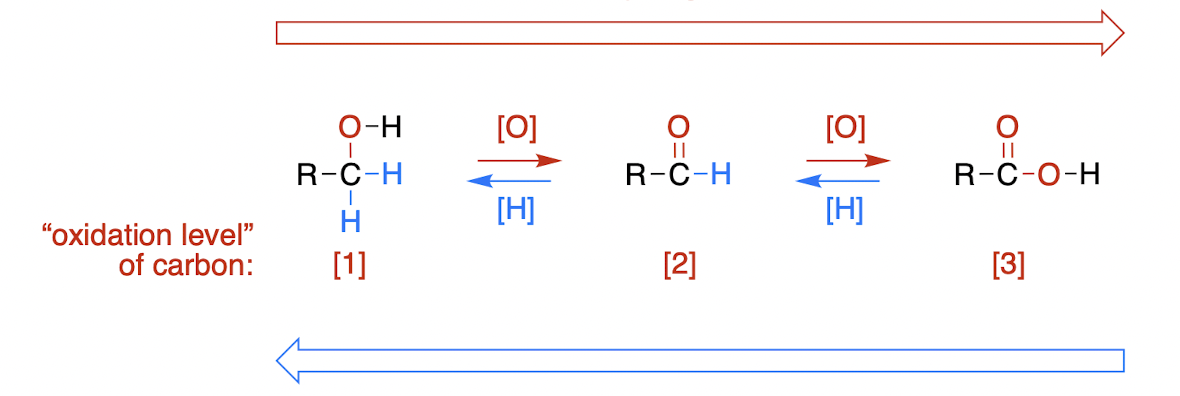
reduction, [H]
a decrease in the number of bonds to electronegative atoms and/or increase in the number of bonds to hydrogen
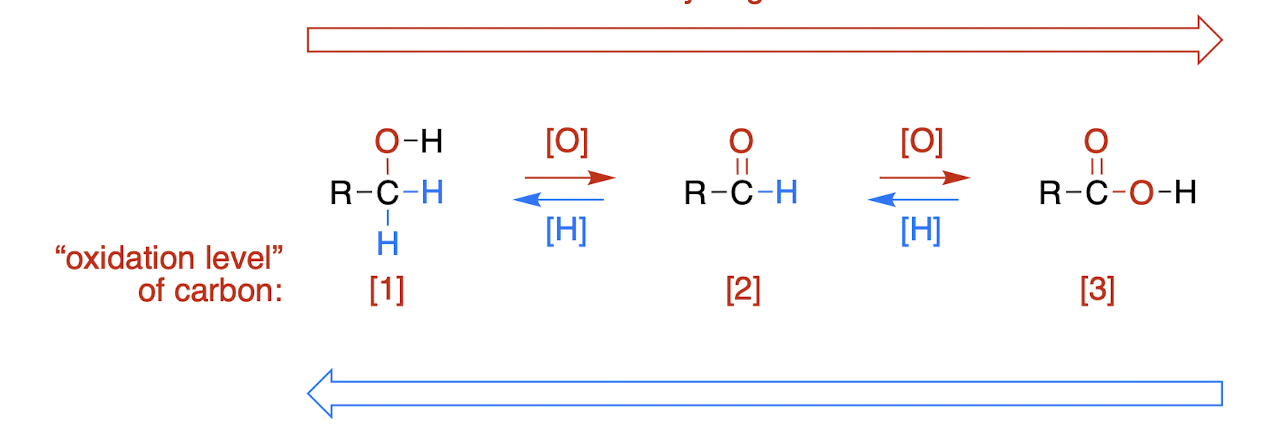
oxidation, [O]
an increase in the number of bonds to electronegative atoms and/or a decrease in the number of bonds to hydrogen
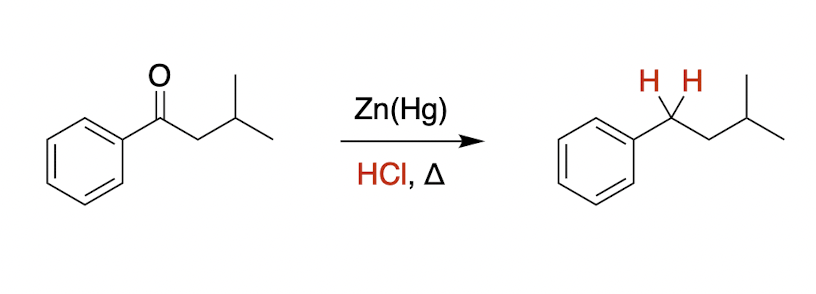
Clemmensen reduction
* only with Class A carbonyl
acidic conditions, no mechanism
class A carbonyl + HCl → deletes C=O bond
what are the catalyst and condition?
Zn(Hg) and Δ
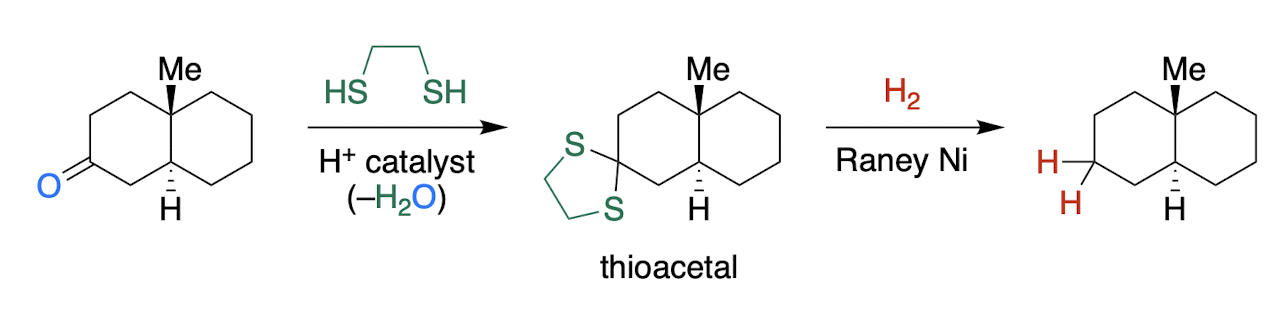
Mozingo Reduction
* only with Class A carbonyl
stepwise thioacetal formation → Raney nickel hydrogenation
general mechanism of alcohol oxidations
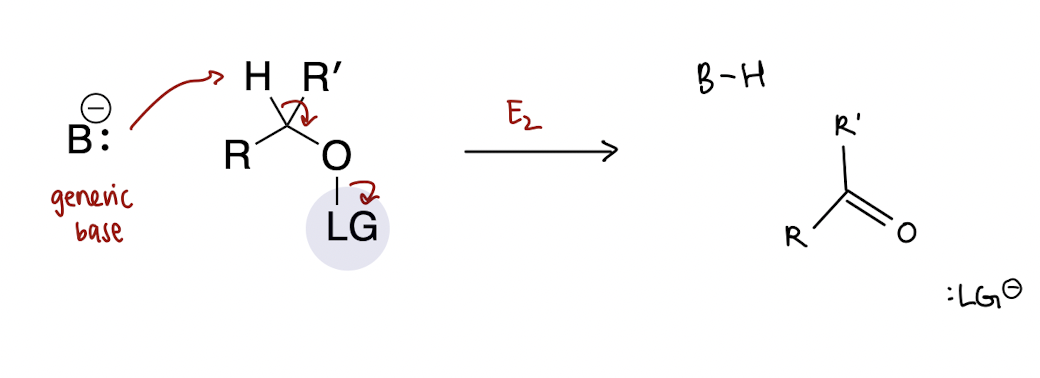
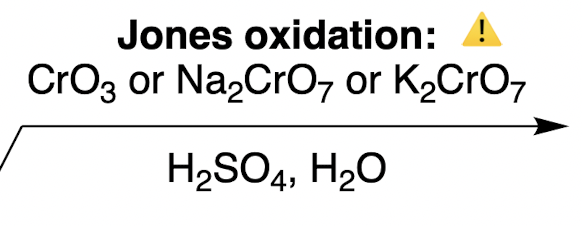
Oxidations of Alcohols: Jones oxidation
1° alcohol + strong harsh condition → ?
carboxylic acid
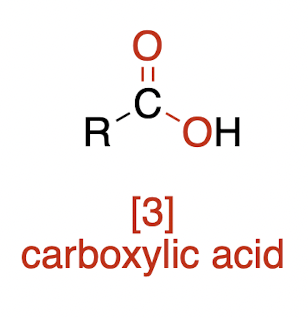
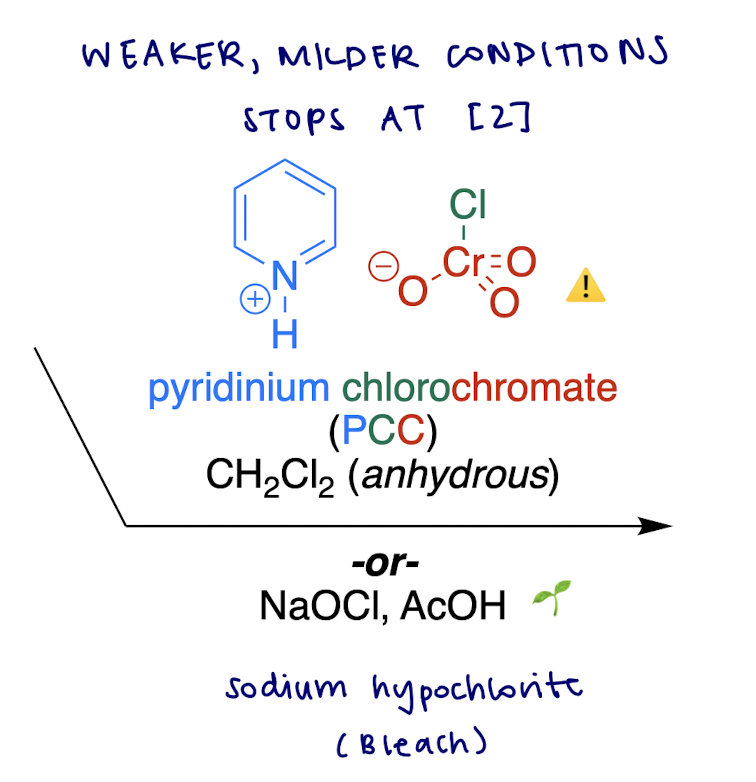
Oxidations of Alcohols
weaker oxidation of 1° alcohol → ?
aldehyde
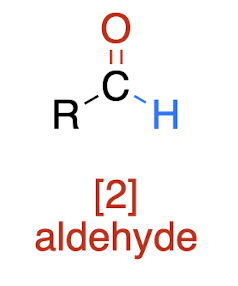
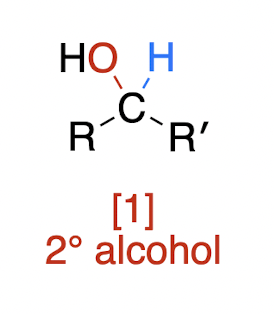
Oxidations of Alcohols
oxiding agents are H2 with Pd,Pd/C,Pt, or Ni catalyst
secondary alcohol + oxidizing agent → ?
ketone
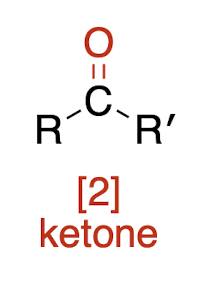
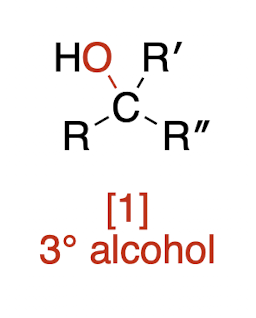
Oxidations of Alcohols
oxiding agents are H2 with Pd,Pd/C,Pt, or Ni catalyst
tertiary alcohol + oxidizing agent → ?
NR—no reaction!!
LiAlH4 and NaBH4 do ___-addition while cuprates do ____-addition
LiAlH4 and NaBH4 do 1,2-addition while cuprates do 1,4-addition
how can we completely delete a C=O bond and replace it with 2 hydrogen atoms? 3 ways
Wolff-Kischner: NH2NH2, KOH, heat
Clemmensen: Zn(Hg), HCl
Mozinga
how can we add water across a double bond? 2 ways
water + acid (Markovnikov)
hydroboration (anti-Markovnikov)
BH3, THF
H2O2, NaOH (aq)
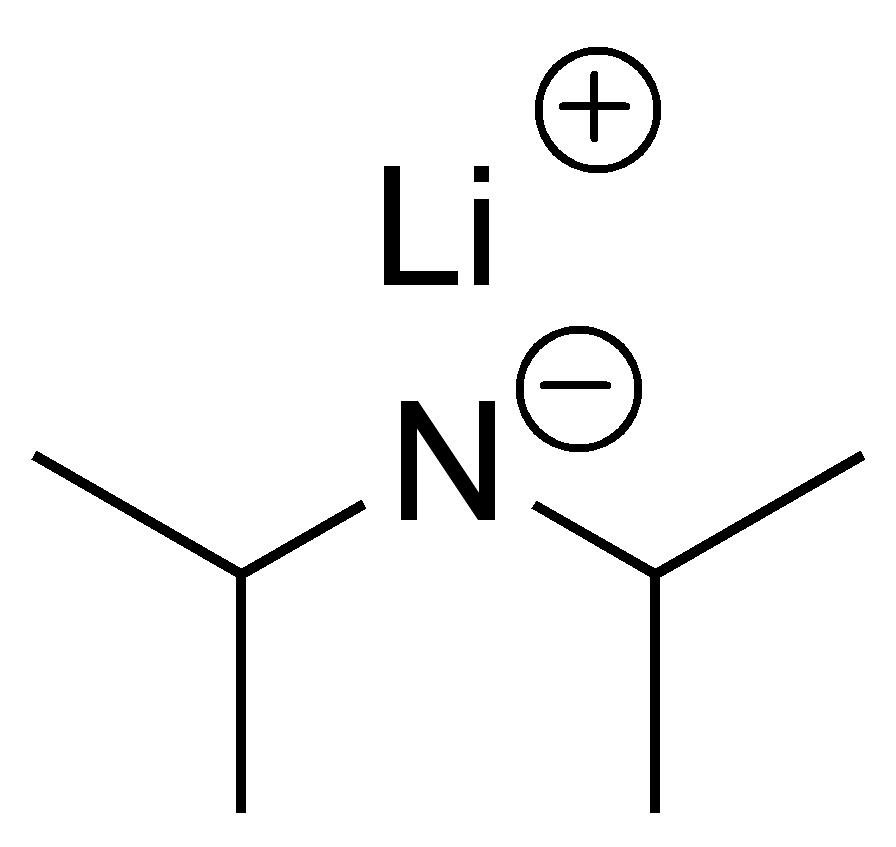
what is LDA?
a bulky strong base that can be used to direct a crossed aldol reaction
C=O → alcohol and nitrile group on the C
KCN, HCN at 0°C
CN → CNH (double bond)
H2 (1 equiv), Pd/C
CN → CNH2 (single bond)
H2 (excess), Pd/C
how do we oxidize an alcohol to class A carbonyl (3 ways)
C—OH → C=O
3 options not three consecutive steps!
PCC
NaOCl, AcOH (bleach and vinegar)
CH2Cl2 (anhydrous)
when does a crossed Aldol reaction occur?
condensation reaction between two different aldehyde or ketone molecules in a protic solvent, like water or alcohol, when the nucleophile and electrophile are different
what reactions are redox-neutral? how can you confirm this
hydrolysis, condensation, and hydration reactions
calculating the oxidation state of relevant carbons and seeing that it doesn’t change from the starting material to the product
how do we oxidize an alcohol to a carboxylic acid (3 ways)
[1] → [3]g
3 options not three consecutive steps!
CrO3
Na2CrO7
K2CrO7
needs to be in H2SO4, H2O