CVS
1/126
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
127 Terms
Valve primarily affected by ARF
Mitral valve (followed by aortic)

ARF is a common complication of …?
Group A strep infection, particularly pharyngitis or other fauces such as scarlet fever, otitis media, etc (1-3 weeks post infection)
Pathogenesis of ARF
Immune mediated delayed response
Antigens of group A strep cross react with cardiac myosin and sacrolemmal membrane proteins (molecular mimicry)
Antibodies produced against them cause damage to endocardium, myocardium, pericardium, heart valves, subcutaneous tissue, tendons, joints, and basal ganglia
Aschoff nodules (composed of multinucleated giant cells surrounded by macrophages and T cells; not seen until subacute or chronic phase) and Anitschkow cells found in heart tissues
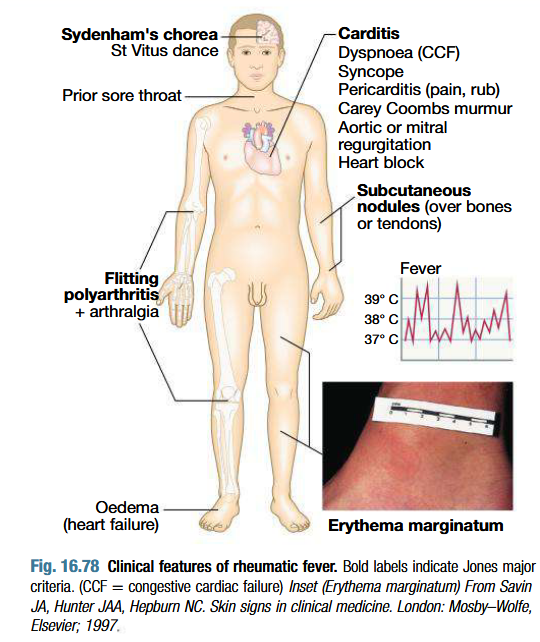
Clinical features of ARF
Fever, anorexia, lethargy and joint pain
5-15 years
Pleurisy, pleural effusion and pneumonia
Pancarditis:
Incidence decreases with age
Breathlessness (due to heart failure or pericardial effusion)
Palpitations or chest pain (due to pericarditis or pancarditis)
Tachycardia
Cardiac enlargement
New or changed murmurs (soft systolic murmur due to mitral regurgitation)
Valvulitis: Carey Coombs murmur (soft mid diastolic murmur)
Pericadial friction rub and precordial tenderness
Arthritis:
Commonest major manifestation
Occurs early when ASO titres are high
Acute painful, asymmetric and migratory inflammation of the large joints (knees, ankles, elbows and wrists)
Joints red, swollen and tender
Jaccoud’s arthritis
Dramatic response to salicylates
Skin lesions:
Erythema marginatum: Nonpruritic serpiginous or annular erythematous rash more prominent on the trunk & inner proximal portions of the extremities.
Red macules that fade in centre but remain red at edges, occuring mainly on the trunk and proximal extremities but not face
Red rings or margins may coalesce or overlap
Subcutaneous nodules which are small (0.5-2.0 cm), firm and painless, best felt over extensor surfaces of bones or tendons - associated with severe carditis
Appear later (3 weeks)
Syndenham’s chorea/ St Vitus dance:
3 months after ARF episode, when all other signs may have gone
Common in women
Emotional lability is first feature
Purposeless, involuntary, choreiform movements of hands, feet or face
Speech may be explosive or halting
Spontaneous recovery
Milk maid grip, pronation of extended hands, wormian movements of tongue
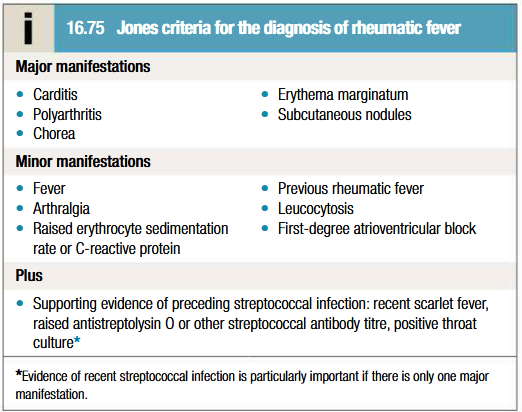
What are the Jones criteria for diagnosis of ARF
When a patient fulfills two major criteria or one major and two minor criteria and meets the absolute requirement for evidence of recent group A strep infection.
List 3 circumstances when ARF is diagnosed w/o strict adherence to Jones criteria
Indolent carditis may be sole manifestation
Chorea may be the sole manifestation
ARF recurrence may not fulfill the Jones criteria
Clinical course of ARF
Only carditis cause permanent cardiac damage.
S/O mild carditis disappear in weeks.
Severe carditis may last for 2 to 6 months.
Arthritis subside within few days to weeks & doesn’t cause permanent damage.
Chorea subsides in 6 to 7 months & does not cause permanent neurologic damage.
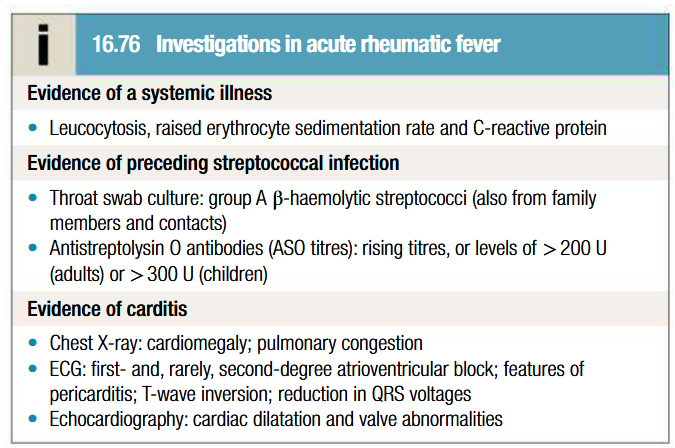
Investigations in diagnosis of ARF
Blood should be taken to measure ESR and CRP (used to monitor disease progress)
Throat cultures +ve only in 10-25% of cases
Serology for ASO antibodies should be done as supportive evidence for diagnosis
Echocardiography shows mitral regurgitation with dilatation of mitral annulus and prolapse of anterior mitral leaflet. May also demonstrate aortic regurgitation and pericardial effusion
ECG: ST and T wave changes
Management of ARF
Aim: limit cardiac damage and relieve symptoms
Bed rest: lessens joint pain and reduces cardiac workload (duration guided by progress of disease). Strenuous exercise to be avoided in patients who’ve had carditis
Treatment of cardiac failure: if unresponsive, valve replacement surgery may be done. AV block may occur occasionally but resolves spontaneously. Rare cases may require pacemaker insertion
Antibiotics: single dose of benzathine benzylpenicilin (1.2 million units IM) or oral phenoxymethylpenicilin (250 mg 4 times daily for 10 days). Erythromycin or cephalosporin to be given is patient is allergic to penicilins. Long term prophylaxis with oral phenoxymethypenicilin (250mg twice daily) or benzathine benzylpenicillin (1.2 million units IM monthly)
Aspirin: for patients with typical migratory polyarthritis and those with carditis without cardiomegaly or CCF. 100 mg/kg/24 hr divided qid PO for 3-5 days, followed by 75 mg/kg/24 hr divided qid PO for 4 wk. Mild toxicity: nausea, tinnitus, deafness. Continued until ESR has fallen
Corticosteroids: For patients with carditis & cardiomegaly or CCF. Prednisone - 2 mg/kg/24 hr for 2-3 wk, followed by a tapering of the dose. At the beginning of the tapering of the prednisone dose, aspirin should be started at 75 mg/kg/24 hr in 4 divided doses for 6 wk.
Supportive: Digoxin, Fluid and salt restriction, Diuretics, Oxygen.
Management of chorea in ARF
Physical and emotional stress should be reduced.
Injection of benzathine penicillin for prophylaxis (indicated as in other rheumatic patients.)
Anti-inflammatory drugs not needed in isolated chorea.
For severe cases-
Phenobarbitone 15 to 30 mg every 6 to 8 hours.
Haloperidol (0.01-0.03 mg/kg/ 24 hr divided bid PO)
Chlorpromazine (0.5 mg/kg q 4-6 hr PO)
Prevention of ARF
Primary:
10 days course of penicilline therapy for streptococcal pharyngitis.
Secondary:
Benzathine penicilline 1.2 million units I.M. every 28 days.
Alternative- Oral penicilline V 250 mg twice daily OR Oral sulfadiazine, 1 gm once daily OR Oral erythromycin 250 mg twice a day.
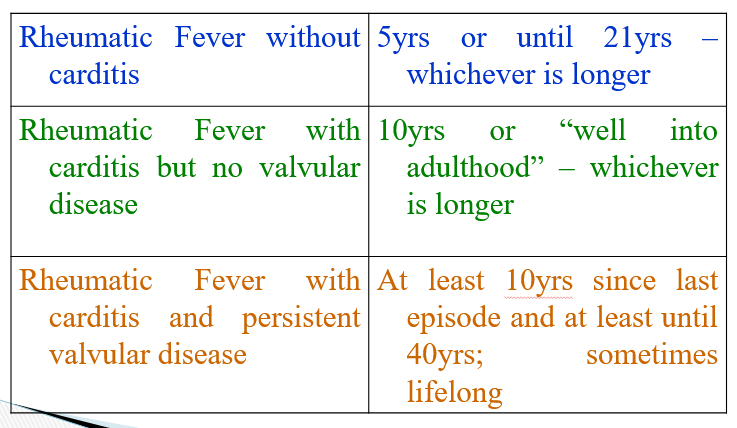
Duration of treatment of ARF
Etiology of mitral stenosis
SLE
Rheumatoid arthritis
Endomyocardial fibrosis
Pure MS occurs in 40% of all patients with RHD and a h/o RF.
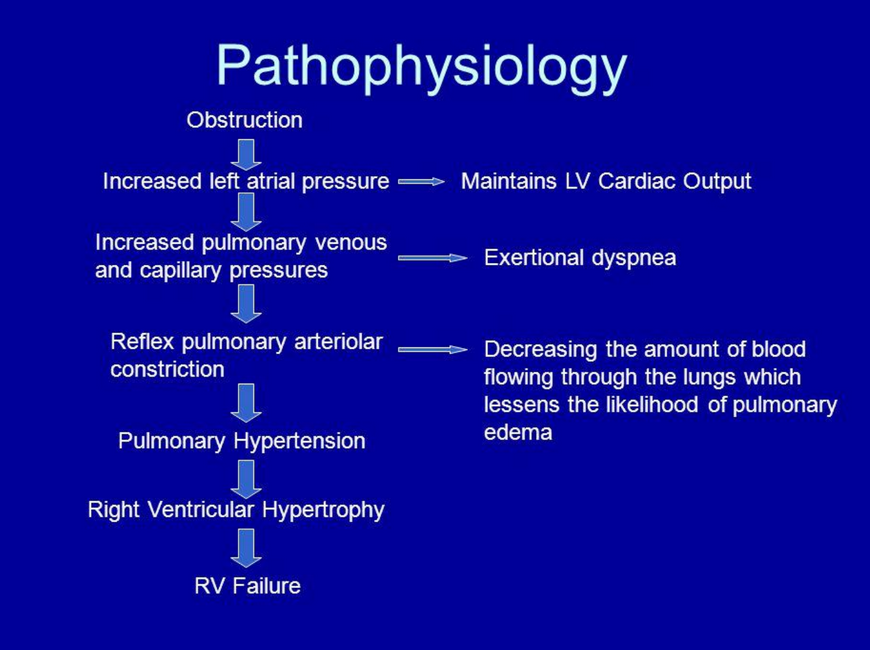
Pathophysiology of mitral stenosis
Mitral valve orifice is slowly diminished by progressive fibrosis, calcification of valve leaflets and fusions of cusps and subvalvular apparatus
As stenosis progresses, left ventricular filling becomes more dependent on left atrial contraction
Dilatation and hypertrophy of LA and LA pressure rises, leading to pulmonary venous congestion + breathlessness
Any increase in HR shortens diastole when mitral valve is open and produces further rise in LA pressure
May lead to atrial fibrillation, which precipitates pulmonary oedema (tachycardia + loss of atrial contraction = haemodynamic deterioration and rapid rise in LA pressure)
If AF is absent: rise in LA pressure is more gradual
Pulmonary hypertension leads to RV hypertrophy and dilatation, triscuspid regurgitation and RHF
Changes in size of mitral valve as stenosis progresses
Normal area of mitral valve is 4-6 cm2
Mild mitral stenosis: 1.5-2.5 cm2
Moderate mitral stenosis: 1.0-1.5 cm2
Severe mitral stenosis: < 1.0 cm2
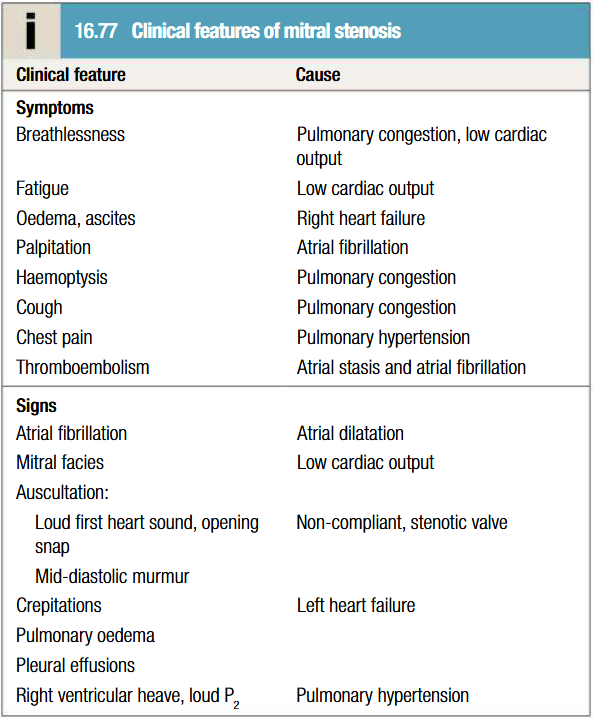
Clinical features of mitral stenosis
Effort related dyspnoea, orthopnoea, PND
Haemoptysis (pink frothy sputum) due to acute pulmonary oedema or pulmonary hypertension
Fatigue due to low cardiac output
Thromboembolism
S1 is loud and palpable: tapping apex beat
Opening snap is audible and moves closer to S2 as stenosis becomes more severe
S1 and opening snap may be inaudible if valve is heavily calcified
Low-pitched mid-diastolic murmur/thrill due to turbulent flow. Murmur accentuated by exercise and during atrial systole
Pre-systolic murmur present earlier in disease
Coexisting mitral regurgitation causes pansystolic murmur, which radiates to axilla
Parasternal lift, systolic murmur and giant v waves in venous pulse due to RV hypertrophy
Pulmonary apoplexy- due to the rupture of thin walled and dilated bronchial veins when there is a sudden increase in left atrial pressure.
Winter bronchitis
Peripheral edema and ascites - Right heart failure
Hoarseness of voice: Dilated LA compressing the recurrent laryngeal nerve (Ortner's syndrome or cardiovocal syndrome)
Malar flush (Mitral facies)
Pulse – Low volume and Irregular
JVP – Prominent ‘a’ wave, Prominent ‘v’ wave, Absent ‘a’ wave
Epigastric pulsation, Left 2nd ICS pulsation
Complications of mitral stenosis
Pulmonary artery hypertension
Right heart failure
Infective endocarditis (rare)
Dysphagia
Laryngeal nerve palsy
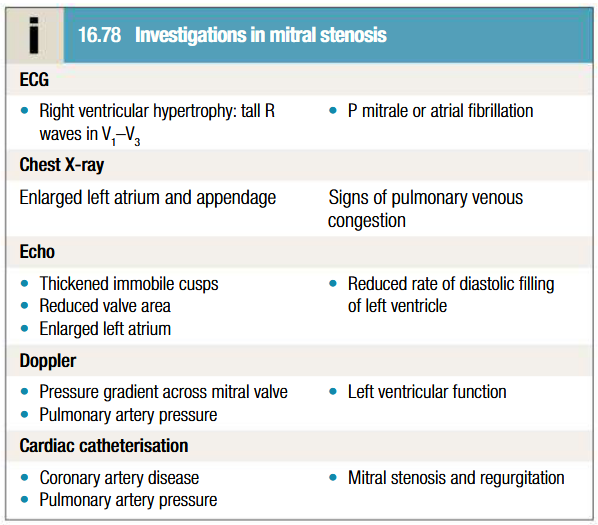
Investigations to be done for mitral stenosis
ECG
CXR: Calcification of the annulus of mitral valve, double atrial shadow, elevation of left bronchus, impingement on esophagus. Stag antler’s sign or Inverted moustache sign in pulmonary hypertension
Echocardiography
Doppler
Cardiac catheterisation
Management of mitral stenosis
Medical management
Anticoagulants to reduce risk of embolism
Digoxin to control ventricular rate
Beta blockers or rate limiting antagonists for AF
Diuretics to control pulmonary congestion
Antibiotic prophylaxis against infective endocarditis (controversial)
Penicillin prophylaxis for secondary prevention of RF
Mitral balloon valvuloplasty
Alternatives: surgical closed or open mitral valvotomy
Follow up at 1-2 yearly intervals
Valve replacement
If there is substantial mitral reflux or valve is rigid and calcified
Mechanical valves (bi-leaflet, tilting disk and ball caged valve) or biological valve
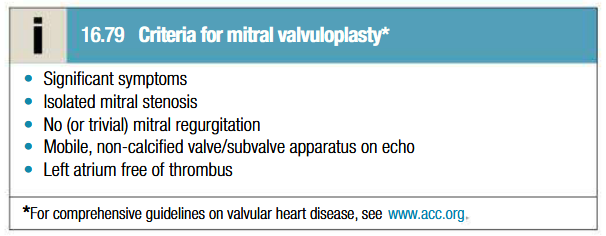
Etiology of mitral regurgitation
Mitral valve prolapse (MVP) – most common
Rheumatic heart disease (20% of RHD)
Infective endocarditis
Ischemic heart disease
Cardiomyopathy
Rheumatoid arthritis
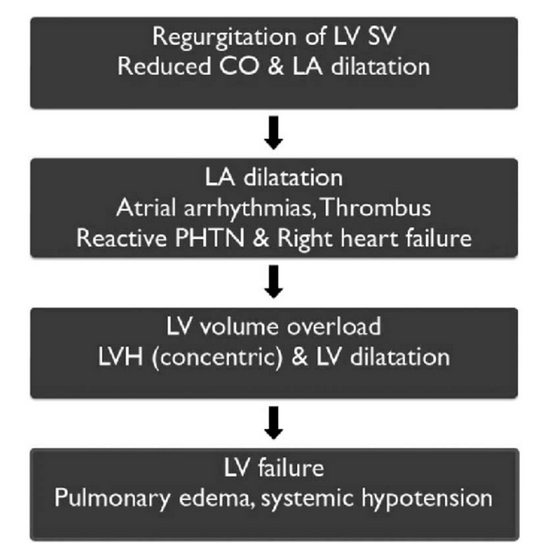
Pathophysiology of mitral regurgitation
Chronic MR causes gradual dilatation of LA (little increase in pressure and few symptoms). LV dilates slowly and LV diastolic and LA pressures gradually increase due to chronic volume overload of LV.
Acute MR causes rapid rise in LA pressure (as LA compliance is normal) and marked symptomatic deterioration
Mitral valve prolapse
Common cause of mild MR
Due to developmental abnormality of mitral valve or degenerative myxomatous change in normal mitral valve
May be associated with Marfan syndrome
Mild: valve remains competent but bulges back into LA during systole, causing mid-systolic click (not always audible) but no murmur
Regurgitant valve: click is followed by late systolic murmur (lengthens as severity increases)
Progressive elongation of chordae tendineae leads to increasing MR and if chordal rupture occurs, severity increases swiftly (rare before 5th or 6th decade)
Associated with arrhythmias, atypical chest pain and small risk of embolic stroke or transient ischaemic attack
Other causes
Dilatation of LV distorts geometry of chordae tendineae and their papillary muscles
Dilated cardiomyopathy and heart faliure from CAD are common causes of functional MR
Endocarditis causes acute MR
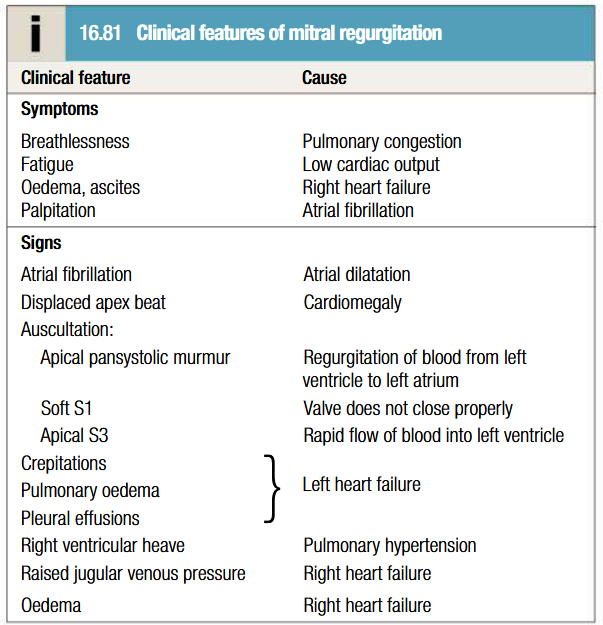
Clinical features of mitral regurgitation
Depends on underlying cause and onset of MR
Symptom complex similar to MS
Sudden-onset MR presents with acute pulmonary oedema
Regurgitant jet causes apical systolic murmur, which radiates to axilla and is accompanied by a thrill
Increased forward flow through valve = loud S3 and short mid-diastolic murmur
Apex beat feals active and rocking due to LV volume overload
Apex beat displaced to the left due to LV dilatation
Chest pain due to LV hypertrophy and pulmonary artery dilatation
BP typically normal
Water hammer pulse
Prominent ‘a’ wave of JVP
Palpable P2
Parasternal and epigastric pulsation
Complications of MR
Left ventricular failure
Atrial fibrillation
Thrombo-embolism
Pulmonary hypertension
Infective endocarditis
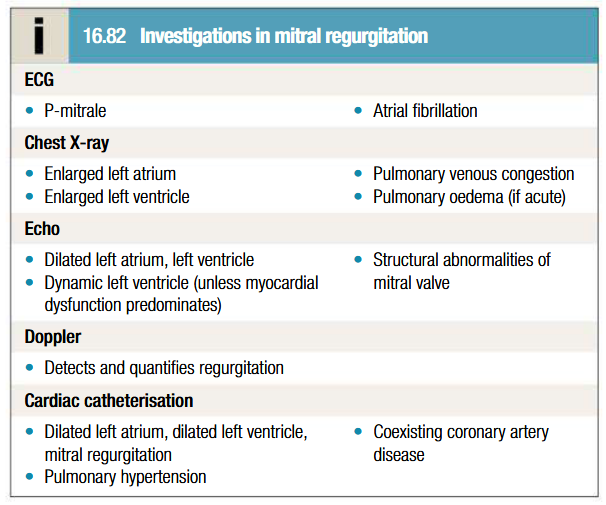
Investigations to be done for MR
ECG shows: Left atrial enlargement, LV hypertrophy
Echo shows: Presence of thrombus or vegetation, severity of MR based on regurgitant jet
Flail MV
Management of MR
Moderate severity: diuretics and vasodilators
AF present: digoxin and anticoagulants
Systemic hypertension present: vasodilators such as ACE inhibitors or ARBs (high afterload worsens MR)
Infective endocarditis prophylaxis
Rheumatic fever prophylaxis
Review patients clinically and by echo
Mitral valve replacement or repair indicated if: progressive cardiomegaly or echocardiographic evidence of deteriorating LV function
Sever MR: mitral valve repair (prevents irreversible LV damage)
MR patients with LVF associated with CAD: if they are to undergo CABG surgery, valve is repaired by inserting annuloplasty ring to overcome annular dilatation and bring leaflets closer together
MV replacement or repair may worsen ventricular function if LV dilatation is underlying cause of MR
What is atrial stenosis
Aortic stenosis (AS) is narrowing of the aortic valve resulting in obstruction of blood flow from the left ventricle to the ascending aorta during systole.
Etiology of atrial stenosis
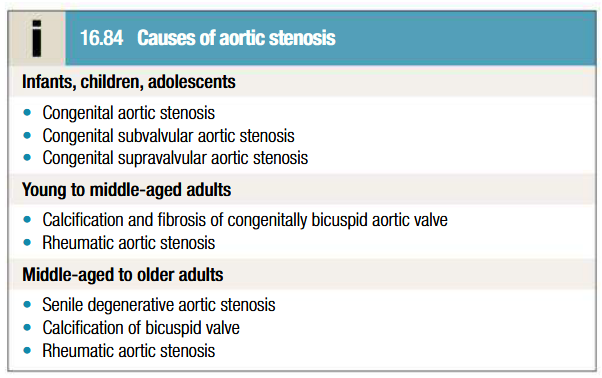
Pathogenesis of aortic stenosis
CO is maintained at cost of steadily increasing pressure gradient across aortic valve
As stenosis progresses: LV becomes increasingly hypertrophied and coronary blood flow may be inadequate to supply the myocardium (such that angina can develop even in absence of coexisting CAD)
Fixed outflow obstruction limits increase in CO needed for exercise
Eventually LV can no longer overcome outflow tract obstruction and LVF happens, leading to pulmonary oedema
Clinical features of aortic stenosis
Cardinal symptomes: SAD (Syncope, Angina, Dypnoea/breathlessness)
Agina: increased demands of hypertrophied LV working against high pressure outflow tract obstruction/presence of coexisting CAD
Dypnoea: cardiac decompensation due to excessive pressure overload placed on LV
Syncope on exertion: CO fails to meet demand leading to fall in BP
Harsh ejection systolic murmur radiates to neck: saw cutting wood and may have musical quality like mew of seagull in older patients
Soft S2 with reverse split (particularly in those with calcified valves)
S4 in some cases
Older patients with non compliant arterial system may have apparantly normal carotid upstrock
Mild cases must be differentiated from aortic sclerosis
Pulsus parvus et tardus (low vol, slow rising and late peaking pulse)
Apico-carotid delay and carotid artery thrill (shudder)
Decrease in systolic BP, low pulse pressure
Sustained/heaving apex beat
Systolic thrill in aortic area
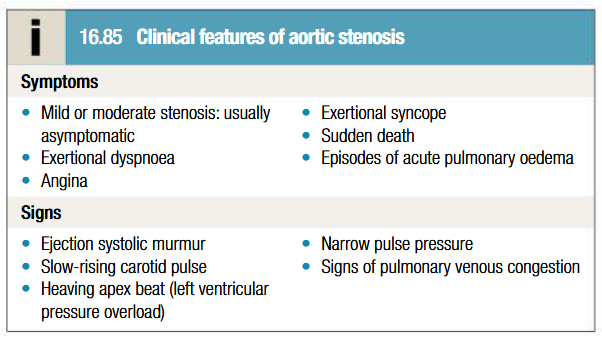
Investigations to be done for aortic stenosis
ECG: Down-sloping ST segments and T inversion (strain pattern) in lateral leads: represents LV fibrosis. May be normal in some cases. AV block due to encroachment of fibrocalcific process on adjacent AV node and His-Purkinje system
Imaging with CT to assess degree of valve calcification
Low flow aortic stenosis
Marked aortic regurgitation and elevated CO may overestimate severity of AS on doppler
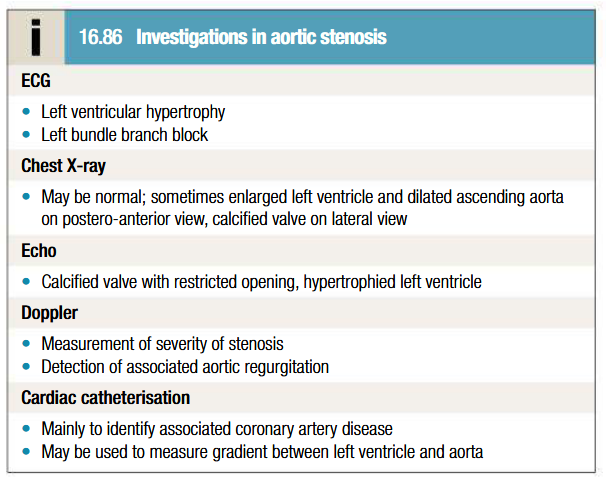
Management of aortic stenosis
Asymptomatic: keep under review (development of cardinal symptoms/evidence of low CO or HF is indication for surgery)
Moderate or severe asymptomatic stenosis: evaluated every 1-2 years (more frquently in older patients with heavily calcified valves) with doppler
Symptomatic severe stenosis: prompt aortic valve replacement (delay = sudden death or irreversible deterioration in LV function). Transcather aortic valve implantation has promising results
Congenital AS: aortic balloon valvuloplasty (not useful in older patients with calcific AS)
AF present/valve replacement with mechainical prosthesis: anticoagulants
Causes of aortic regurgitation
Chronic AR
Bicuspid aortic valve or disproportionate cusps
Rheumatic diseases: SLE, rheumatoid arthritis
Aortic dilatation due to Marfan syndrome, Aneurysm, syphilis, ankylosing spondylitis
Acute AR
Infective endocarditis
Aortic dissection
Trauma
Pathogenesis of AR
Regurgitation of blood through aortic valve causes LV to dilate as CO increases to maintain demand
Stroke vol of LV may eventually be doubled and major arteries are then conspicuously pulsatile
LVF develops as disease progresses
Rise in LV end-diastolic pressure and pulmonary oedema occur as a consequence
Clinical features of aortic regurgitation
Patient may be asymptomatic for 10-15 years
Until onset of breathlessness, awareness of heart beat, particularly when lying on left side may be only symptom (due to increased stroke vol)
Dyspnea: Orthopnea and PND
Palpitations
Nocturnal angina
Water hammer pulse
Increased systoli BP and decreased diastolic BP
Peripheral oedema
Classical signs may be masked when features of HF predominate by tachycardia and abrupt rise in LV end diastolic pressure
Hyperdynamic diffuse apex beat
Diastolic thrill at aortic area
S3 in severe AR or LVF
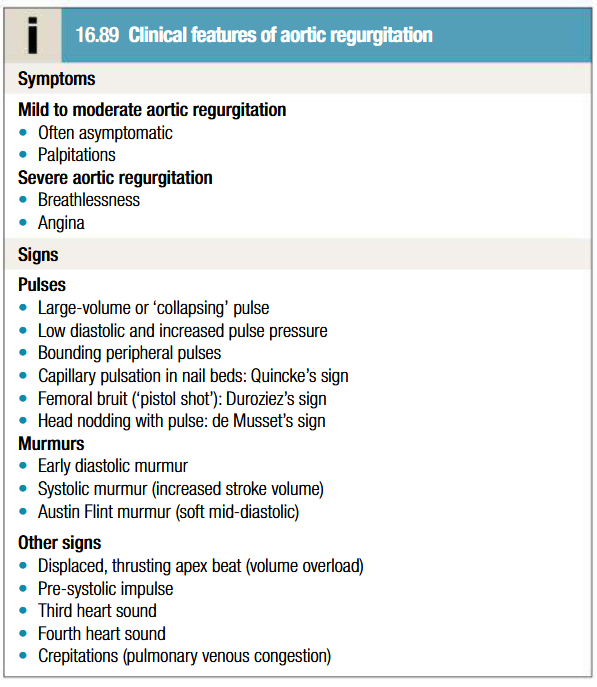
Mumurs heard in aortic regurgitation
Early-diastolic murmur: high-pitched blowing sound, loudest at the left sternal border, decrescendo, best heard in expiration, in sitting and leaning forward position.
Austin-Flint murmur at the cardiac apex in severe AR → low-pitched, mid-diastolic rumbling murmur due to blood jets from the AR striking the anterior leaflet of the mitral valve
Peripheral signs of AR
Becker sign - pulsations of the retinal arterioles
Corrigan sign – prominent carotid pulsation
de Musset sign - Bobbing motion of the patient's head with each heartbeat
Hill sign – (Popliteal cuff systolic blood pressure 20 mm Hg higher than brachial cuff systolic blood pressure) 20-40mmHg – mild AR
40-60mmHg – mod AR
> 60mmHg – Severe AR
Duroziez sign - Systolic murmur over the femoral artery with proximal compression of the artery, and diastolic murmur (Duroziez murmur) over the femoral artery with distal
Light-house sign – Alternate flushing & blanching of forehead
Landolfi’s sign - Change in pupil size
Gerhardt’s sign - pulsations of spleen
Rosenbach’s sign - pulsations of liver
Quincke sign - pulsations of the fingernail bed with light compression of the fingernail
Traube sign ("pistol-shot" sign) - Booming systolic and diastolic sounds auscultated over the femoral artery
Investigations done for aortic regurgitation
MRI can also be done to assess degree and extent of aortic dilatation if the latter is suspected
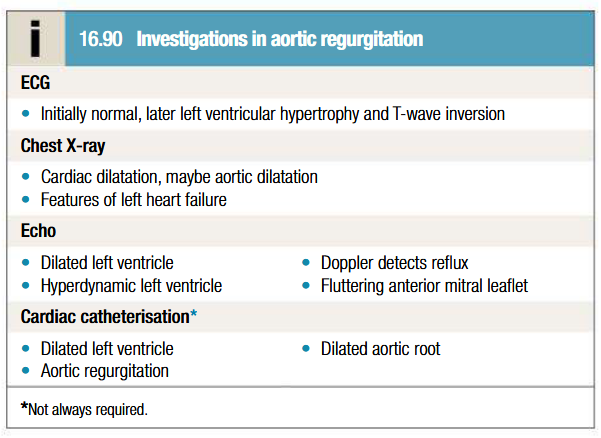
Management of aortic regurgitation
Long term vasodilator therapy in asymptomatic patients with severe AR (Hydralazine, Nifedipine, ACE inhibitors), particularly if systemic hypertension is present
Follow up on asymptomatic patients annually with echo for evidence of increasing ventricular size (aortic valve replacement may be considered if LVEF ≤ 50% - controversy)
Digoxin and diuretics (furosemide, spironolactone) in HF patients
Infective endocarditis prophylaxis and antibiotic therapy for syphilis
Aortic valve replacement is indicated if AR causes symptoms, may need to be combined with aortic root replacement and coronary bypass surgery
If aortic root dilatation is cause of AR, aortic root replacement is advised
What is Eisenmenger syndrome
In patients with severe and prolonged pulmonary hypertension: left-to-right shunt may reverse
Results in right-to-left shunt and marked cyanosis
Cyanosis may be more apparent in feet and toes than upper part of body (differential cyanosis)
More common with large ventricular septal defects or PDA
Patients are at particular risk from abrupt changes in afterload that exacerbate right-to-left shunting (eg: vasodilatation, anaesthesia and pregnancy)
Long term prognosis is poor
Pathophysiology of persistent ductus arteriosis
During fetal life, blood from pulmonary artery passes through ductus arteriosus into aorta
Persistence of duct causes a continuous AV shunt from aorta to pulmonary artery (as pressure in aorta is higher)
Vol of shunt depends on size of duct
A good percentage of the LV output may be reciculated through the lungs with a consequent increase in work of heart
Large left-to-right shunt in infancy may cause considerable rise in pulmonary artery pressure leading to progressive pulmonary vascular damage
Clincal features of PDA
Small shunts: no symptoms for years
Large ductus: growth and development may be retarded
No disability in infancy but cardiac failure may eventually ensure
Dyspnea is first symptom
Continuous machinery murmur heard with late systolic accentuation, maximal in 2nd left ICS below clavicle. Accompanied by thrill
Pulses are increased in vol
If shunt reverses (Eisenmenger syndrome), murmur becomes quieter, may be confined to systole or disappear
Investigations to be done to PDA
ECG: usually normal, may show evidence of RV hypertrophy
Echo: specific views, such as through the suprasternal notch may be needed to reveal the defect
Management of PDA
Duct can be closed at cardiac catheterisation with an implantable occlusive device
Closure should be done in infancy if shunt is significant and pulmonary resistance is not elevated
Closure may be delayed to later in childhood for smaller shunts
PG synthetase inhibitor (ibuprofen or indometacin) may be used in 1st week of life to induce closure
In cases with impaired lung perfusion (such as in severe pulmonary stenosis and left-to-right shunt), it may be advisable to improve oxygenation by keeping ductus patent with PG treatment
Treatments don’t work if ductus is intrinsically abnormal
Pathogenesis of coarctation of aorta
Narrowing of aorta occurs in region where ductus arteriosus joins aorta (at isthmus just below origin of left subclavian artery)
Causes raised BP affecting vessels of head and neck proximal to coarctation
Reduced BP and impaired circulation distally
Clinical features of coarctation of aorta
Male predilection
Associated with other abnormalities such as bicuspid aortic valve and berry aneurysms of cerebral circulation
May occur as complication of Takayasu’s disease
Important cause of cardiac failure in newborns; symptoms absent in older kids
Headaches from hypertension proximal to coarctation
Weakness or cramps in legs due to decreased ciculation
Femoral pulses are weak and delayed in comparison to radial pulse
Systolic murmur heard posteriorly, over coarctation
Ejection click and systolic murmur in aortic area may be present due to bicuspid aortic valve
Localied bruits due to formation of collaterals (mainly involve periscapular, internal mammary and intercostal arteries)
Investigations to be done for coarctation of aorta
Imaging by MRI is investigation of choice
CXR in early childhood is often normal, later shows changes in contour of aorta (indentation of descending aorta: 3 sign) and notching of undersurfaces of ribs from collaterals
ECG: evidence of LV hypertrophy, confirmed via echo
Management of coarctation of aorta
Untreated: death due to LVF, dissection of aorta or cerebral haemorrhage
Surgical correction is adivsed in all but mildest forms (if done early, persistent hypertension can be avoided)
If repaired later in life: patient often remains hypertensice or develops recurrent hypertension later on
Recurrence of stenosis may occur as child grows: corrected via balloon dilatation and stenting
Coexistent bicuspid aortic valve may lead to progressive AS or AR, and requires long term follow up
Pathogensis of ASD
Normal RV is more compliant than LV
Patent foramen ovale is associated with shunting of blood from LA to RA and then to RV and pulmonary arteries
Gradual enlargement of right side of heart and pulmonary arteries
Pulmonary hypertension and shunt reversal sometimes complicate ASD, but are less common and occur later in life
Clinical features of ASD
Female predilection
Many kids are asymptomatic for years
Dypnoea
Cardiac failure
Arrhythmias, especially AF
Large shunt: diastolic flow murmur over tricuspid valve (high-pitched)
Characteristic physical signs due to vol overload of RV
Wide (due to delay in RV ejection), fixed (septal defect equalises left and right atrial pressures) splitting of S2
Systolic murmur over pulmonary valve
Types of ASD
Ostium primum: defect in atrioventricular septum and is associated with cleft mitral valve
Ostium secondum: more common; involves foramen ovalis/ovale
Investigations to be done for ASD
Echo is diagnositc: demonstrates defect and shows RV dilatation, RV hypertophy and pulmonary artery dilatation
TOE: precise size and location of defect can be seen
CXR: enlargment of heart and pulmonary artery, pulmonary plethora
ECG: incomplete RBBB (RV depolarisation is delayed due to ventricular dilatation). Left axis deviation in primum defect
Management of ASD
Pulmonary flow ratio of 1.5:1 present: close defect surgically
Smaller defects can be managed conservatively and patients monitorred regularly via echo
Closure can be done at cardiac catheterisation using implantable closure devices (good long term prognosis, unless pulmonary hypertension develops)
Severe pulmonary hypertension and shunt reversals: DO NOT OPERATE
Pathogenesis of VSD
Incomplete septation of ventricles
Embryologically, interventricular septum has a membranous and muscular portion, latter is further divided into inflow, trabecular and outflow portions
Most congenital defects are perimembranous, occuring at junction of membranous and muscular portions of septum
Clinical features of VSD
Most common congenital cardiac defect
Flow from high pressure LV to low pressure RV during systole: pansystolic murmur, heard best at left sternal border but radiating all over pericordium
Small defect = loud murmur (maladie de Roger) in absence of any other hemodynamic disturbance
Large defect = quieter murmur (particularly if RV pressure is elevated)
Found immediately after birth or when shunt reverses
May result in cardiac failure in infants
In some infants: murmur becomes quieter and disappears due to spontaneous closure of defect
If cardiac failure complicated large defect, it only becomes apparent in first 4-6 weeks of life
Prominent parasternal pulsation
Tachypnoea
Indrawing of lower ribs on inspiration
Investigations to be done for VSD
Echo: identifies even small defects which may not be haemodynamically significant and are likely to close spontaneously
Patients with larger defects: monitor via serial echo to check for signs of pulmonary hypertension
CXR in large defects: pulmonary congestion
ECG in large defects: bilateral ventricular hypertrophy
Management of VSD
Small defects: no treatment needed
Cardiac failure in infancy: digoxin, diuretics and sometimes ACE inhibitors
Presisting cardiac failure: surgical repair of defect
Percutaneous closure devices are under development
Pulmonary hypertension is developing: surgical repair
Eisenmenger syndrome: heart-lung transplantation (surgical closure is contraindicated)
Long term prognosis is good unter Eisenmenger syndrome develops
What does tetralogy of fallot consist of
RV hypertrophy
Larger VSD and overriding aorta
Ventricular outflow tract obstruction
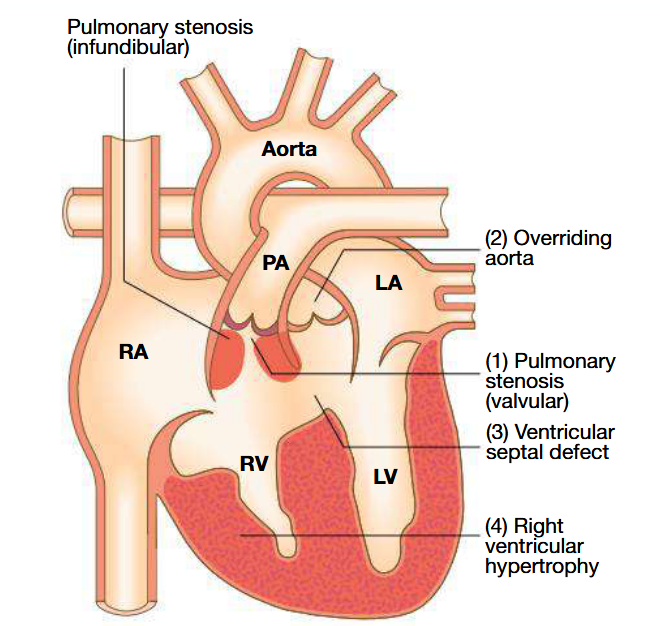
Pathogenesis of tetralogy of fallot
Result of abnormal development of bulbar septum that separates ascending aorta from pulmonary artery
Normally, it aligns and fuses with outflow part of interventricular spetum
RV outflow obstruction is most often subvalvular (dynamic and may increase suddenly with sympathetic stimulation)
VSD is large and similar in aperture to aortic orifice
Results in elevated RV pressure and right-to-left shunting of cynotic blood across VSD into aorta
Clinical features of teratology of fallot
Kids usually cyanosed (may not be in neonates (acyantoic TOF) as large shunt develops only when RV pressure rises to equal or exceed LV pressure)
Child may become suddenly cyanosed (typically after feeding or crying) and may become apnoeic and unconscious
Older kids: cyanotic spells are uncommon but cyanosis becomes increasingly apparent + stunting of growth + digital clubbing + polycythemia
Kids may obtain relief by squatting after exertion (fallot’s sign) to increase afterload of left heart and reduce shunting
Loud ejection systolic murmur in pulmonary areas (as for pulmonary stenosis)
Investigations to be done for tetralogy of fallot
Echo is diagnostic: demonstrates aorta is not continuous with anterior ventricular septum
ECG: RV hypertrophy
CXR: abnormally small pulmonary artery and boot shaped heart
Management of tetralogy of fallot
Total correction of defect by surgical relief of pulmonary stenosis and closure of VSD
Primary surgical correction may be done prior to age of 5 years
If pulmonary arteries too hypoplastic for surgical repair: palliation in form of Blalock-Taussig shunt (anastomosis is created between pulmonary artery and subclavian artery to improve pulmonary blood flow and artery development)
Follow up to identify residual shunting, recurrent pulmonary stenosis and arrhythmias
Implantable defibrillator may be recommended in adulthood
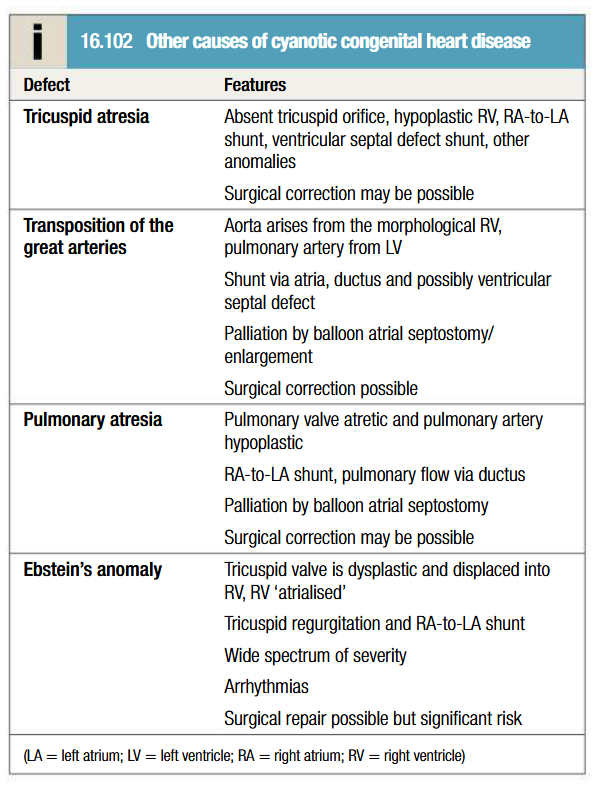
List some causes of cyanotic congenital heart diseases
Pathology of infective endocarditis
Occurs at sites of pre-existing endocardial damage (particularly virulent organisms can cause endocarditis in previously normal heart)
May follow IV drug use, congenital heart conditions (VSD, MR and AR)
Areas of endothelial damage attract deposits of platelets and fibrin that are vulnerable to colonisation by blood born microbes (which are then protected from host defence mechanisms via the same)
Vegetations composed of organisms, fibrin and platelets grow and may become large enough to cause obstruction/embolism
Adjacent tissues are destroyed and abscesses may form
Valve regurgitation may develop/increase if valve is damaged by tissue distortion, cusp perforation or disruption of chordae
Extracardiac manifestations: vasculitis, skin lesions, mycotic aneurysms, spleen/kidney infarcts
Micro-organisms involved in infective endocarditis
Subactute: Viridans streptococci (Strep. mitis and Strep. sanguis), Enterococcus faecalis, E. faecium, Strep. gallolyticus
Acute: Staph. aureus, Step. pneumoniae, Strep. pyogenes
Post-op: Staph. epidermidis and other coagulase negative staphylococci
Q fever endocarditis: Coxiella burnetii (history of contact with farm animals)
HACEK (Haemophilus aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae)
Brucella endocarditis (history of contact with goats or cattle)
Yeasts and fungi (candida and aspergillus)
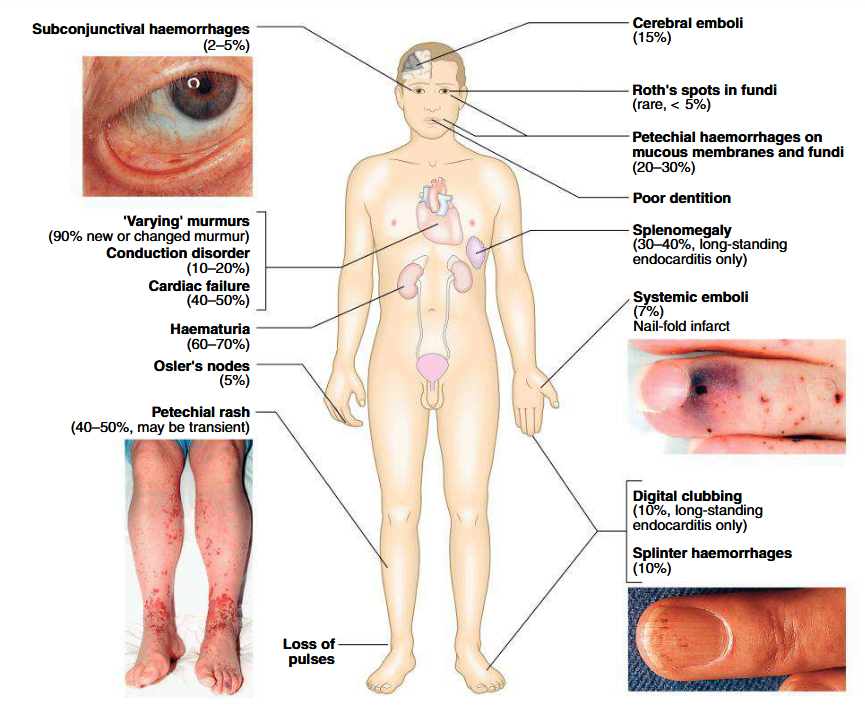
Clinical features of subacute endocarditis
Suspected when patients with congenital or valvular heart diseases develop persistent fever, complains of unusual tiredness, night sweats or weight loss, or develops new signs of valve dysfunction or heart failure
May present as embolic stroke or peripheral arterial embolism
Purpura and petechiae
Splinter haemorrhages under fingernails or toenails
Osler’s nodes are painful, tender swellings at finger tips (sign of vasculitis)
Digital clubbing is a late sign
Spleen is frequently palpable
Coxiella infection: spleen and liver are enlarged
Non-visible hematuria
Clinical features of acute endocarditis
Severe febrile illness
Prominent and changing heart murmurs
Petechiae
Clinical stigmata of chronic endocarditis typically absent
Embolic events are common
Cardiac or renal failure may develop
Abscesses may be detected on echo
Partially treated acute endocarditis behaves like subacute endocarditis
Clinical features of post-op endocarditis
Unexplained fever in patient who has had heart valve surgery
Infection involves valve ring and may resemble acute/subacute endocarditis
Mobidity and mortalility is high; revision surgery is needed
Duke’s criteria of infective endocarditis diagnosis
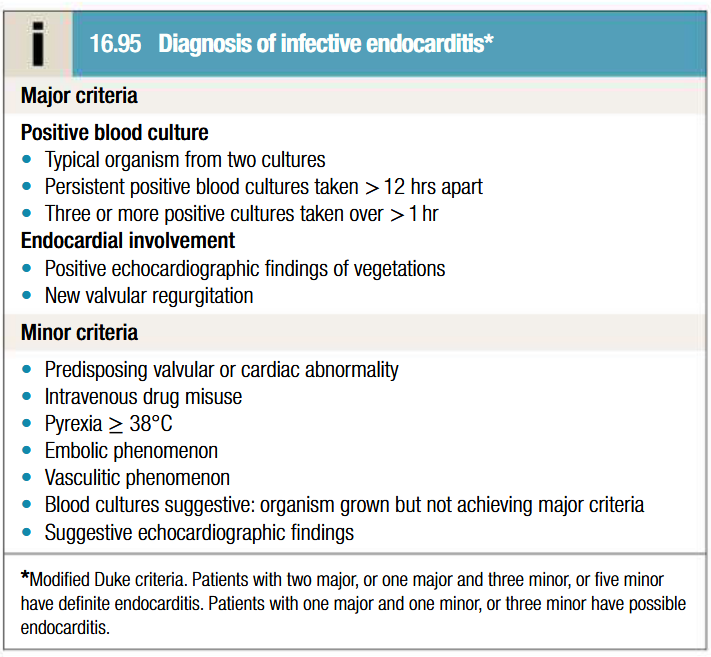
Investigations to be done for infective endocarditis
Blood culture: 3-6 sets should be taken prior to commencing therapy, from peripheral sites at intervals ≥ 6 hours (reduces risk of misdiagnosis). Both aerobic and anaerobic cultures are to be done
Echo: detecting and following progress of vegetations, assessing valve damage and detecting abscess formation
ESR: elevated
Normocytic normochromic anemia
Leukocytosis
Proteinuria
Measurement of serum CRP to monitor progress
ECG: may show development of AV block (due to aortic root abscess formation) and infarction due to emboli
CXR: evidence of cardiac failure and cardiomegali
Management of infective endocarditis
Multidisciplinary approach: physician, surgeon and microbiologist all working together
Source of infection to be removed asap (eg tooth with apical abscess should be extracted)
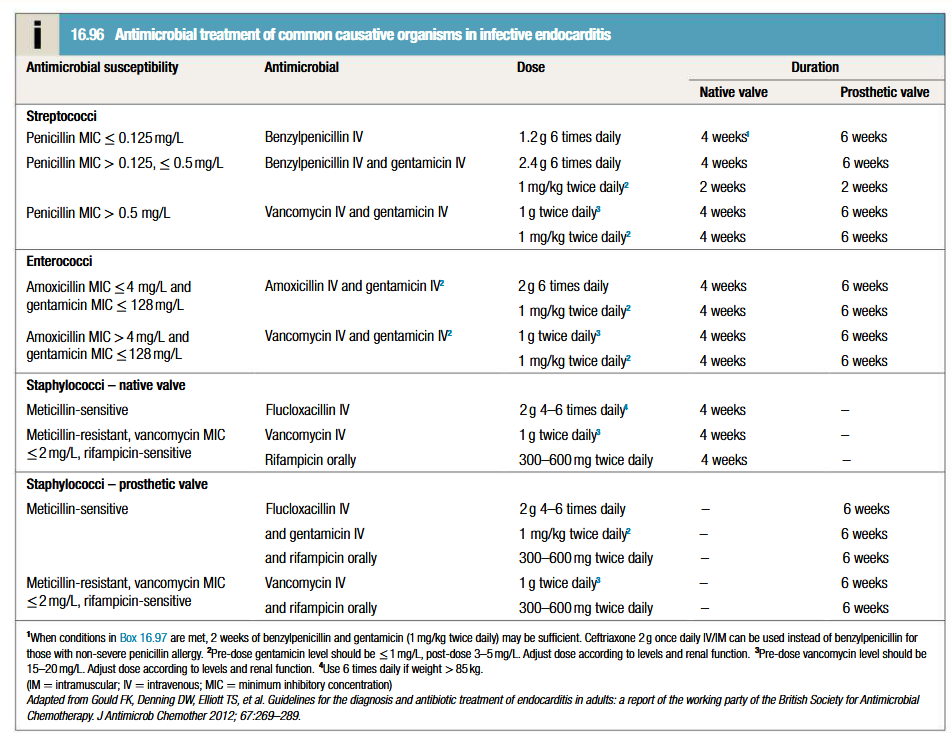
Empirical treatment: depends on presentation/suspected organism/presence of prosthetic valve/allergies.
Subacute: antibiotic therapy should be withheld till blood culture results, otherwise start amoxicilin (2g IV 6 times daily, w/ or w/o gentamicin)
Acute: empirical therapy with vancomycin (1g IV twice daily) and gentamicin (1 mg/kg IV twice daily), with dose adjustment based on antibiotic levels
Prosthetic valve endocarditis: vancomycin and gentamicin at same doses as for acute + rifampicin orally (300-600 mg twice daily)
Following identification of organism, MIC for the same should be determined to guide therapy
2 week treatment period may be sufficient for fully sensitive strains of streptococci, provided specific conditions are met (eg: native valve, MIC < or = 0.125 mg/L, no vegetations > 5 mm, no evidence of poor prognostic factors or thromboemboli)
Cardiac surgery with debridement may be required with particularly agressive organisms (Staph. aureus, fungi)
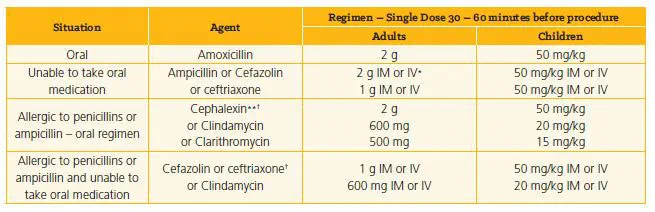
Infective endocarditis prophylaxis
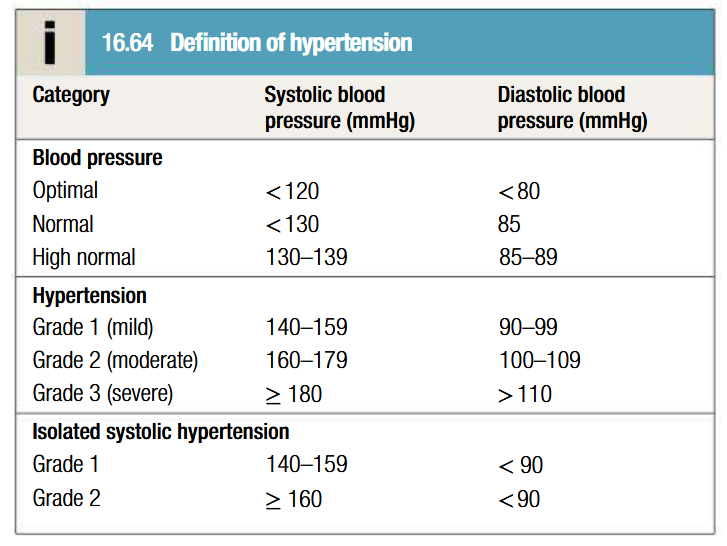
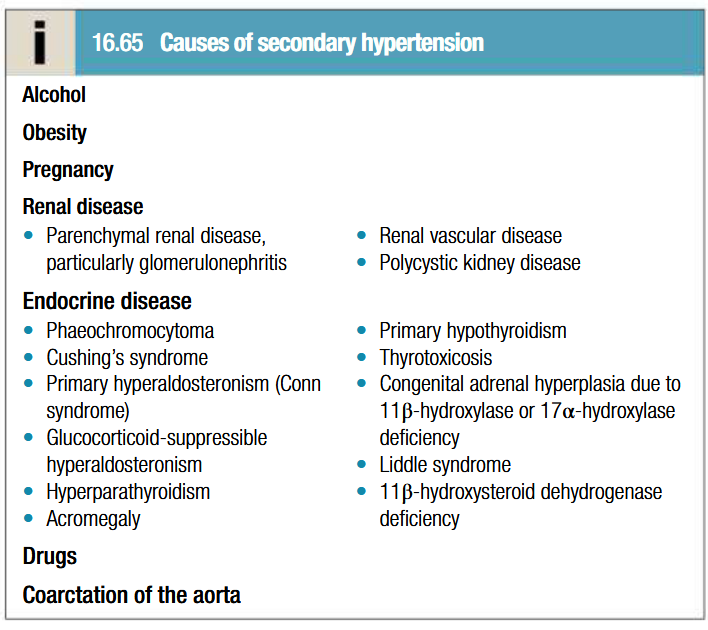
What is hypertension
Pathogenesis of hypertension
In more cases, no specific underlying cause can be found (essential hypertension)
Age is a strong risk factor
Environmental factors: high salt intake, heavy consumption of alcohol, obesity and lack of exercise
Impaired intrauterine growth and low birth weight are laso associated with an increased risk of hypertension
In larger arteries: internal elastic lamina is thickened, smooth muscle hypertrophied and fibrous tissue is deposited. Vessels dilate, become tortuous and less compliant
In smaller arteries: hyaline arteriosclerosis occurs in the wall, lumen narrows and aneurysms may develop. Widespread atheroma leading to coronary and cerebrovascular disease
Structural changes in vasculature perpetuate and aggravate hypertension by increasing PVR and reducing renal blood flow = activates RAAS system
Clinical features of hypertension
Usually asymptomatic (hence BP check every 5 years is advisable to patients over 40 years)
Radio-femural delay in patients with coarction of aorta
Enlarged kidneys in patients with polycystic kidney disease
Abdominal bruits in patients with renal artery stenosis
Moon facies and characteristic habitus of Cushing’s syndrome
Examination: risk factors such as central obesity and hyperlipidaemia
Signs of complications of hypertension: LV hypertrophy, accentuation of aortic component of S2 and presence of S4
AF due to diastolic dysfunction caused by LV hypertrophy or CAD
LVF due to severe hypertension
Examination of optic fundi: cotton wool exudates due to retinal ischaemia or infarction (fade in few weeks). Hard exudates (small, white, dense deposits of lipid) and microaneurysms (dot haemorrhages) = diabetic retinopathy
Central retinal vein thrombosis
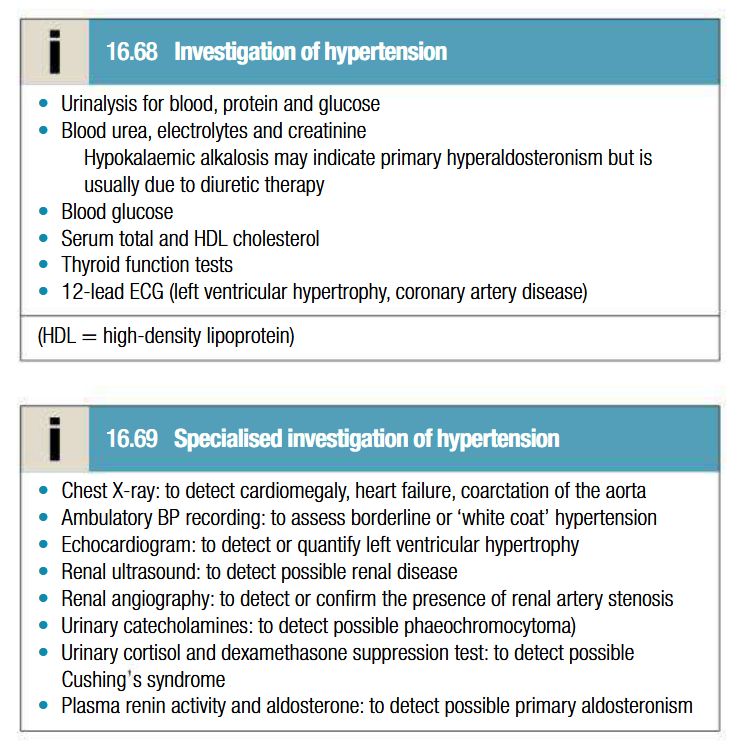
Investigations to be done for hypertension
BP measurement:
To be made to nearest 2 mmHg, in sitting position with arm supported and repeated after 5 mins of rest if 1st recording is high
Obese patients: cuff should contain a bladder that encompasses at least 2/3 of arm circumference
Ambulatory BP measurement:
Series of automated ambulatory BP measurements obtained over 24 hrs provide better profile and correlate more closely with evidence of target organ damage than casual BP measurements
Systematically lower than clinic measures
Day time avg should be used to guide treatment
Home BP measurement:
Useful in those with unsually labile BP, refractory hypertension, symptomatic hypotension and white coat hypertension
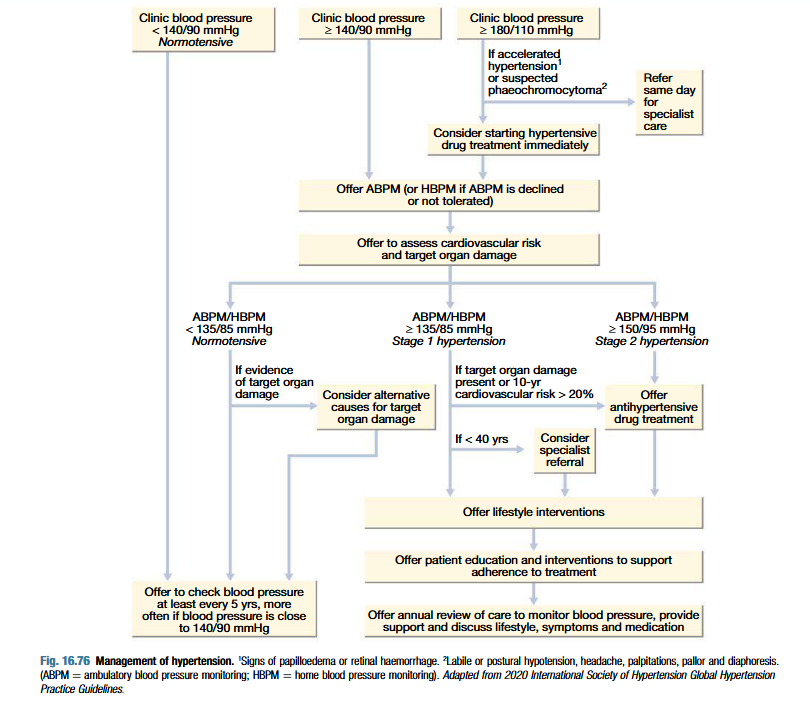
Management of hypertension
Intervention thresholds:
Based on systolic and diastolic BP
Patients with diabetes or cardiovascular diease have lower threshold
Optimum BP for reduction of major cardiovascular events = 139/83 mmHg (lower in diabetic patients)
Non-drug therapy:
Lifestyle changes: useful in those with borderline hypertension
Correcting obesity, reducing alcohol intake, restricting salt intake, regular exercise and increasing consumption of fruits and veggies
Stopping smoking, eating oily fish and maintaining diet low in saturated fat
Drug therapy:
Thiazides: 2.5 mg bendroflumethiazise or 0.5 mg cyclopenthiazide daily
ACE inhibitors: enalapril 5-40 mg daily, or ramipril 5-10 mg daily or lisinopril 10-40 mg daily (may precipitate renal failure in patients with renal artery stenosis)
ARBs: irbesartan 75-300 mg daily or valsartan 40-160 mg daily
Calcium channel antagonists: amlodipine 5-10 mg daily or nifedipine 30-90 mg daily (particularly useful in older people)
Beta-blockers: no longer first line therapy (unless patient has angina). Metoprolol 100-200 mg daily, atenolol 50-100 mg daily or bisoprolol 5-10 mg daily
Combined beta and alpha blockers: labetalol 200 mg-2.4 g daily in divided doses or carvedilol 6.25-25 mg daily
Other vasodilators: prazosin 0.5-20 mg daily in divided doses, indoramin 25-100 mg twice daily or doxazosin 1-16 mg daily
Aspirin
Statins: patients with established vascular disease or hypertension with high risk of developing cardiovascular disease
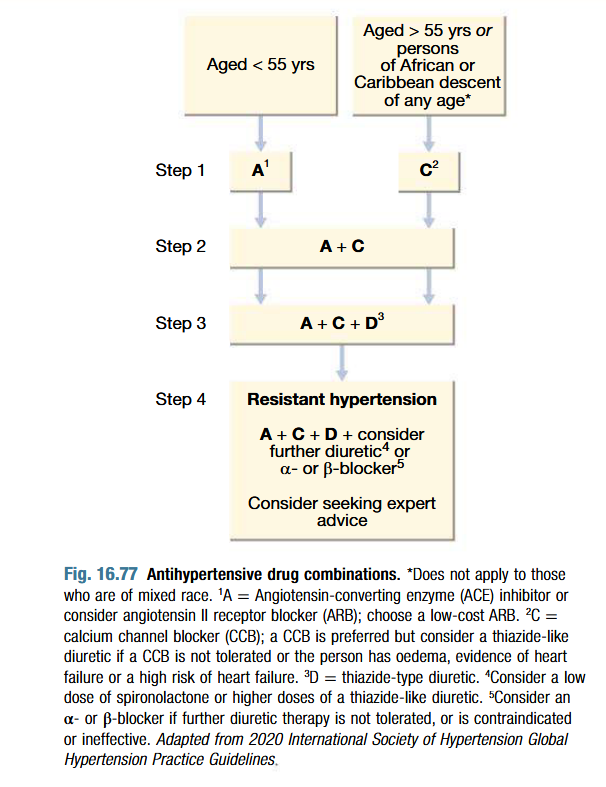
How to choose an antihypertensive
What is refractory hypertension
Situation wherein multiple drug treatments do not give adequate control of BP
Usually do to non-adherence to therapy but can also be due to resistence (perhaps due to failure to recognise underlying cause)
Spironolactone is a particularly useful addition in patients with treatment resistant hypertension
What is accelerated hypertension
Characterised by accelerated microvascular damage with fibrinoid necrosis in the walls of small arteries and arterioles and by intravascular thrombosis
LVF and death may follow
Diagnosis:
Evidence of high BP
Rapidly progressive end-organ damage (such as retinopathy grades 3-4, renal dysfunction or/and hypertensive encephalopathy)
Management:
Lowering BP too quickly may lead to altered autoregulation and cause cerebral damage and precipitate coronary or renal insufficiency
Controlled reduction to 150/90 mmHg over 24-48 hrs is ideal
Can be achieved in most patients via oral drug therapy and bed rest
IV or IM labetalol 2mg/min to max of 200 mg
IV GTN 0.6-1.2 mg/hr
IM hydrazaline 5-10 mg aliquots repeated at ½ hourly intervals
IV sodium nitroprusside 0.3-1.0 microgram/kg bodyweight/min
Pathogenesis of CAD
Cause in most patients: atherosclerosis
Other causes: aortitis, vasculitis and autoimmune connective tissue diseases
Atherosclerosis:
Progressive, inflammatory disorder of arterial wallCharacterised by focal lipid rich deposits of atheroma that remain clinically silent until they become large enough to impair tissue perfusion/until ulceration and disruption of lesion results in thrombotic occluslion
Begins early in life, with lipid depositing at sites of altered arterial shear stress (eg: bifurcations). Associated with abnormal endothelial function at said site
High risk: cigarette smokers and familial hyperlipidaemia and hypertension
During evolution of this plaque, monocytes and other inflammatory cells bind to receptors expressed by endothelium
These cells migrate into intima and pick up oxidised LDL particles by phagocytosis to become foam cells
Extracellular lipid pools appear in the intimal space where foam cells die and release their contents
In response to cytokines, smooth muscle cells migrate from media into intima and change from contractile to fibroblastic phenotype: stabilises plaque by covering lipid core with smooth muscle cells and matrix
If inflammation predominates: plaque becomes active or unstable and may be complicated by ulceration and thrombosis (due to release of cytokines by macrophages)
Exposure of plaque content to blood will trigger platelet response
May induce +ve or -ve remodelling
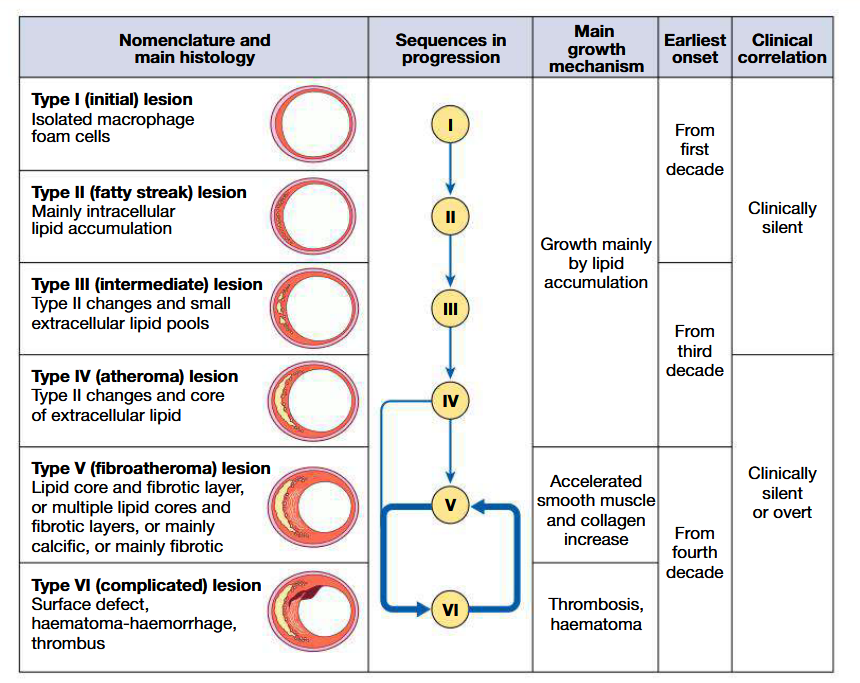
List risk factors for atherosclerosis
Age and sex: Pre-menopausal women have lower rates than men
Genetics
Smoking: especially in younger patients
Hypertension: incidence increases as BP increases
Hypercholesterolaemia: risk rises with serum cholestrol level
Diabetes mellitus: especially type 2 DM. Associated with a diffuse disease that is difficult to treat
Haemostatic factors: platelet activation and high plasma fibrinogen concentrations are associated with increased risk
Physical activity: regular exercise has a protective effect
Obesity: particularly if central or truncal
Alcohol: excess consumption increases risk
Diet: diets deficient in fresh fruit, vegetables and polyunsaturated fatty acids are associated with increased risk
Personality: certain traits are associated with increased risk
Social deprivation
Management of CAD
Primary prevention
Population based strategy: aims to modify risk factors of whole population through diet and lifestyle advice (eg: legislation that prevents smoking in public places has been associated with reduced MI rates)
Targeted strategy: for high-risk individuals. Absolute risk of atheromatous cardiovascular disease should be considered before initiating treatment
Secondary prevention:
Involves targeting interventions at individuals with cardiovascular disease
Clinical manifestations of CAD
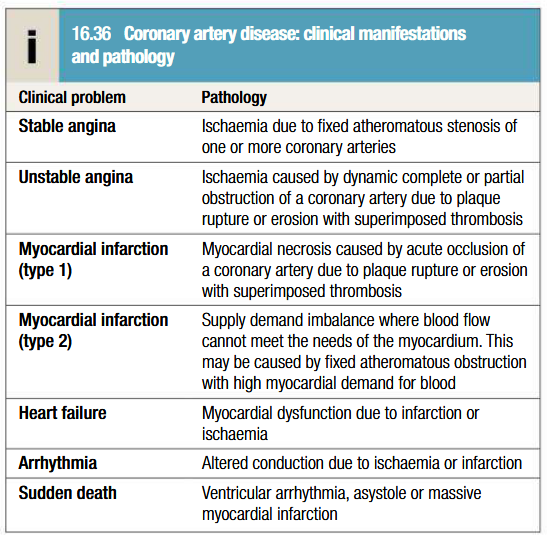
Pathogenesis of angina pectoris
Coronary atherosclerosis most common cause
Coronary artery spasm: may result in prinzmetal’s angina (ST elevation on ECG)
Syndrome X: constellation of typical angina and normal coronary arteries on angiography with objective evidence of MI on stress testing. Subset of patients have impaired myocardial vasodilatory reserve = microvascular angina
Other causes: aortic stenosis, hypertrophic obstructive cardiomyopathy and aortitis
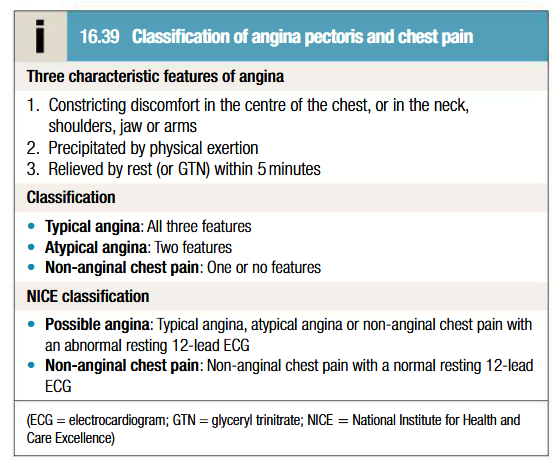
Classification angina pectoris and chest pain
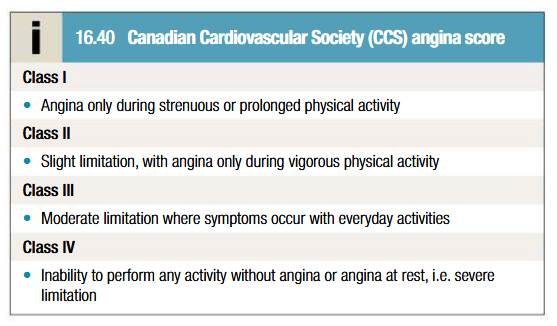
What is Canadian Cardiovascular Society (CCS) angina score
Clinical features of angina pectoris
History is most important factor
Stable angina is categorised as typical angina, atypical angina or non-anginal chest pain
Warm-up angina: discomfort arises when patient starts walking but doesn’t come later despite greater effort
Physical exam may be unremarkable
Search for ejection systolic murmur (aortic stenosis and hypertrophic obstructive cardiomyopathy), important risk factors (hypertension, DM), LV dysfunction (cardiomegaly, gallop rhythm) and other manifestations of arterial disease (carotid bruits, PAD)
Look for unrelated conditions that may exacerbate angina (anemia, thyrotoxicosis)
Investigations to be done for angina pectoris
Stress-testing and non-invasive imaging to confirm diagnosis
Exercise ECG using standard treadmill or bicycle ergometer protocol, while monitorring pulse, BP and general condition: planar or downslope ST segment depression of 1 mm or more indicatice os ischaemia. Up-sloping ST depression less specific (may give false +ve wtih digoxin therapy, LVH, bundle branch block, etc)
CT coronary angiography: clarifies diagnosis and guides therapy (as well as invasive cardiac catheterisation); gives info about extent and nature of CAD (performed when coronary artery bypass graft surgery or percutaneous coronary intervention is being considered)
Known CAD patients: myocardial perfusion scanning or stress echo is indicated (perfusion defect at rest and not at stress = reversible MI, whilst perfusion defect in both phases = previous MI)
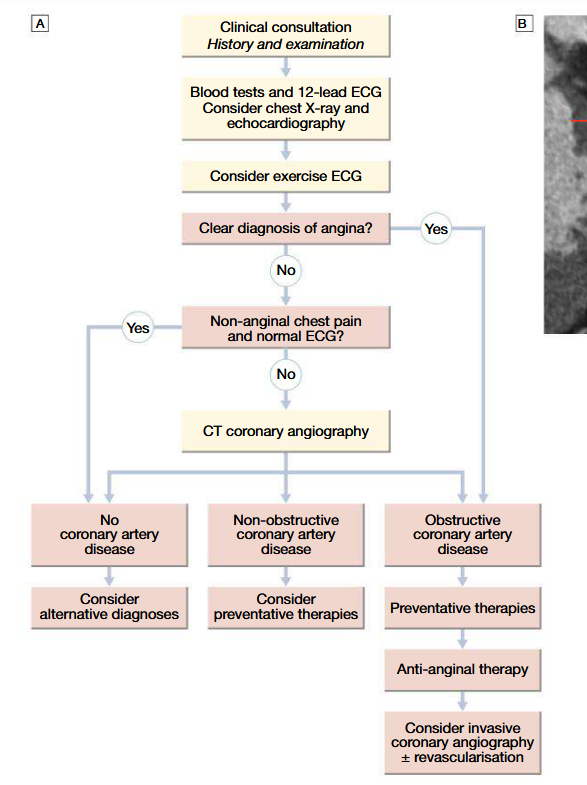
Management of angina (drug therapies)
Education and advise on lifestyle changes
All patients with angina secondary to CAD should recieve low dose aspirin therapy (75 mg) to be continued indefinitely (Clopidogrel 75 mg daily is an alternative if aspirin causes side effects). Prescribe statins too
Anti-anginal drug therapy: usually started with sublingual GTN and beta blocker, then add calcium channel blocker or long term nitrate as needed
Nitrates: act directly on vascular smooth muscle to produce venous and arteriolar dilatation (hence reducing preload and afterload). Sublingual GTN (MDI 400 microgram per spray) indicated for acute attacks. Prolonged therapeutic approach involves administering GTN transcutaneously via patches (5-10 mg daily) or as a slow release buccal tablet (1-5 mg, 4 times daily). Isosorbide dinitrate (10-20 mg thrice daily) and isosorbide mononitrate (20-60 mg once or twice daily) can be given orally
Beta blockers: reduce HR, BP and contractility, but may provoke bronchospasm in asthamatics. Once daily slow release metoprolol (50-100 mg) or bisoprolol (5-15 mg). Do not withdraw drugs abruptly (withdrawl syndrome)
Calcium channel antagonists: reduce BP and contractility. Dihydropyridine calcium antagonists (Nifedipine 5-20 mg thrice daily or amlodipine 2.5-10 mg daily) may cause reflex tachycardia and hence should be combined with beta blockers. Verapmil (40-80 mg thrice daily) and diltiazem (60-120 mg thrice daily) can be used as monotherapy. May aggravate HF in patients with poor LV function
Potassium channel activators: nicorandil (10-30 mg twice daily orally) acts as a vasodilator
If channel antagonist: Ivabradine (2.5-5 mg twice daily orally) modulates ion channels in the sinus node causing bradycardia. Safe in HF patients
Ranolazine: 375 mg twice daily, inhibits late inward sodium current in coronary artery smooth muscle cells, with effects on calcium flux and vascular tone
Non-pharmacological treatments of angina
Percutaneous coronary intervention
A fine guide wire is passed across coronary stenosis under radiographic control and used to position a balloon, which is then inflated to dilated the stenosis
May be combined with deployment of a coronary stent
Excellent symptom control, but doesn’t reduce MI or improve survival in patients with chronic stable angina
Palliative therapy for patients with recurrent angina post coronary artery bypass grafting
Complications: occlusion of coronary artery by thrombus or loose flap of intima, mild myocardial damage, restenosis (due to elastic recoild and smooth muscle proliferation; within 3 or so months)
Adjunctive therapy with potent platelet inhibitor such as P2Y12 receptor antagonists (clopidogrel, prasugrel or ticagrelor) in combination with aspirin and heparin
Coronary artery bypass grafting:
Internal mammary arteries, radial arteries or reversed segments of patients own saphenous vein can be used
Post op angina due to graft failure (due to technical problems or poor run off due to disease in distal native coronary vessels)
Late recurrence: due to progressive disease in native vessels or graft degeneration
Arterial grafts have better long term patency
Aspirin (75-150 mg daily) and clopidegrol (75 mg daily) improve graft patency
Neurological complications are common
What does acute coronary syndrome encompass
Unstable angina: characterised by new-onset or rapidly worsening angina (crescendo angina), angina on minimal exertion or angina at rest in absence of myocardial injury
MI
Risk markers indicative of poor prognosis of acute coronary syndrome
Recurrent ischaemia
Extensive ECG changes at rest or during pain
Raised plasma troponin I or T conc.
Arrhythmias
Haemodynamic complications (hypotension, mitral regurgitation)
Classification and criteria for diagnosis of MI
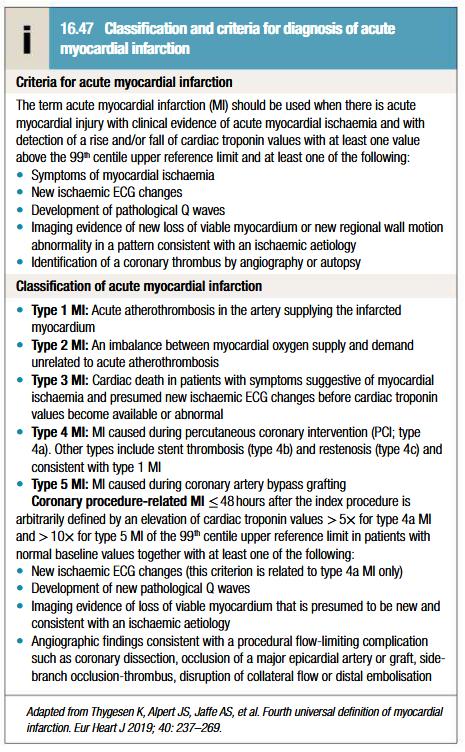
Criteria for diagnosis of previously unrecognised MI
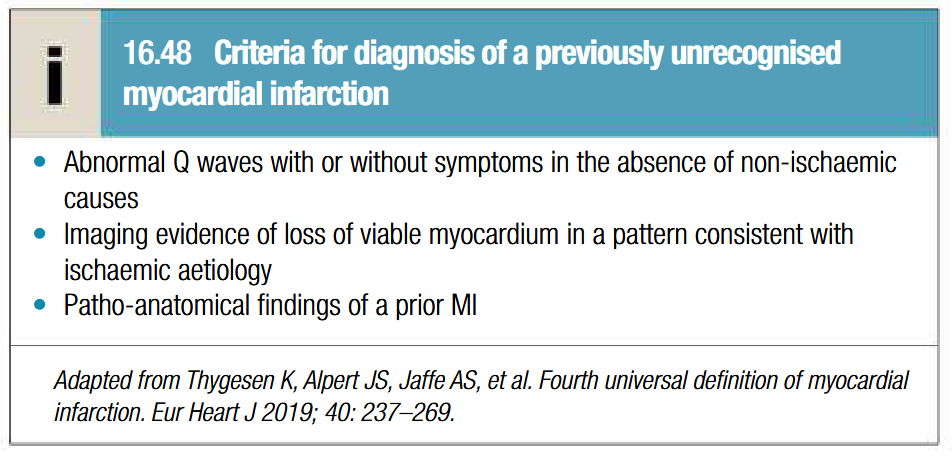
Clinical features of acute coronoary syndrome
Chest pain at rest: same sites as angina but more severe and lasts longer (described as tightness, heaviness or constriction in chest)
Breathlessness, vomitting and collapse
Painless or silent MI is common in older patients or those with DM
Sinus bradycardia due to vagal stimulation in inferior MI
Nausea and vomitting may be aggravated by opiates
MI may occur in absence of physical signs
Sudden death from ventricular fibrilation or asystole
Complications of acute coronary syndrome: arrhythmia
Common but often transient and or no haemosynamic or prognostic significance
Risk minimised by adequate pain relief, rest and correction of hypokaelaemia
VF is thought to be a major cause of death if left untreated
Prompt defibrillation restores sinus rhythm and is life saving
Presence of ventricular arrhythmias during convalescent phase of ACS may be marker of poor ventricular function and may hearld sudden death
Patients may benefit from electophysiological testing and specific anti-arrhythmic therapy
AF is common and doesnt require emergnecy treatment (unless it causes a rapid ventricular rate with hypotension or ciculatory collapse)
Digoxin or beta blocker usually treatment of choice for AF
AF may be associated with LVF
Bradycardia may occur but doesnt need treatment
Inferior MI may be complicated by AV block (usually temporary)
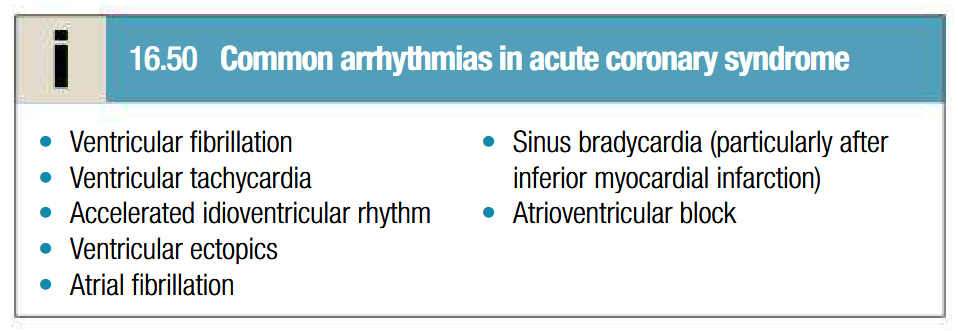
Complications of acute coronary syndrome: recurrent angina
Prompt coronary angiography with a view to revascularisation
Dynamic ECG changes and ongoing pain = treat with IV glycoprotein IIb/IIIa receptor antagonists (tirofiban 400 ng/kg/min for 30 min then 100 ng/kg/min for 48 hrs OR abciximab initially 180 microgram/kg, then 2 micogram/kg/min for upto 72 hrs)
Resistant pain or marked haemodynamic changes = intra-aortic balloon counterpulsation and emergency revascularisation
Post-infarct angina may occur in patients treated with thrombolysis (due to residual stenosis) = consider for early (within 24 hrs) coronary angiography with a view to coronary revascularisation
Complications of acute coronary syndrome: acute heart failure
Reflects extensive myocardial damage
Poor prognosis
All other complications more likely to occur when HF is present
Complications of acute coronary syndrome: pericarditis
Particularly common on 2nd and 3rd days following infarct
Patient may recognsise a different pain has developed at the same site (positional and tends to be worse or sometimes present only on inspiration)
Audible pericardial rub
Opiate based analgesics may be used
NSAIDS and steroidal anti-inflammatory drugs increase risk of aneurysm and myocardial rupture in early recovery period hence AVOID
Complications of acute coronary syndrome: Dressler syndrome
Persistent fever
Pericarditis
Pleurisy
Probably due to autoimmunity
Symptoms occur few weeks or months after MI and subside within days
Prolonged or severe symptoms = treat with high dose aspirin, NSAIDS or glucocorticoids