GCSE AQA Trilogy Separate Science Chemistry: Atoms
0.0(0)
Card Sorting
1/19
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
1
New cards
Compound
A substance made up of atoms of two or more different elements joined by chemical bonds. For example, water.
2
New cards
Element
A pure substance made of only one kind of atom For example, a diamond is made of pure carbon!
3
New cards
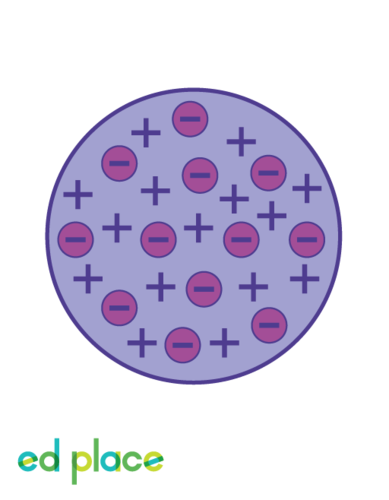
Plum Pudding Model (Thomson)
JJ Thomson's atomic model which depicts a positively charged space containing negatively charged electrons
4
New cards
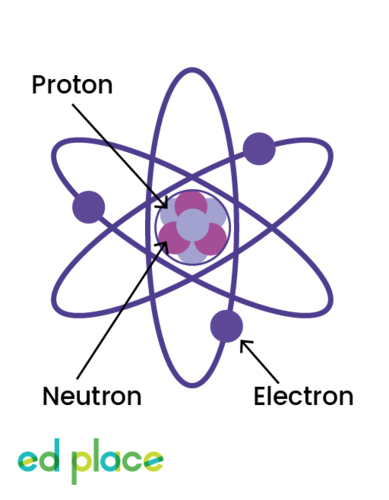
Electrons
Negatively charged particles
5
New cards
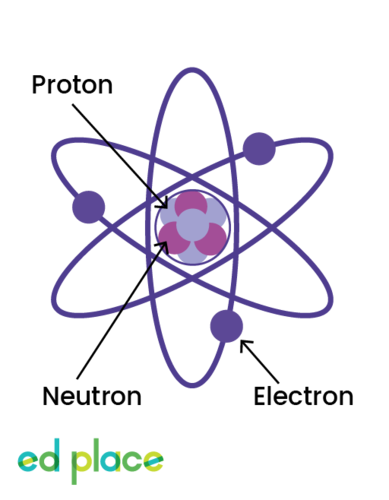
Proton
A particle that has a positive charge and is found in the nucleus of an atom
6
New cards
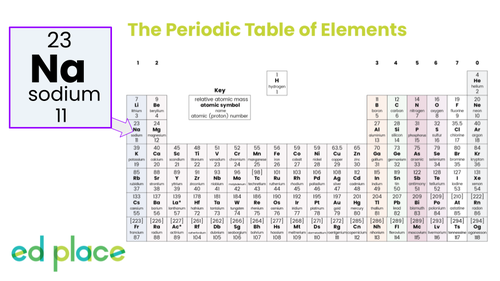
Sodium
Atomic Number: 11
Symbol: Na
Symbol: Na
7
New cards
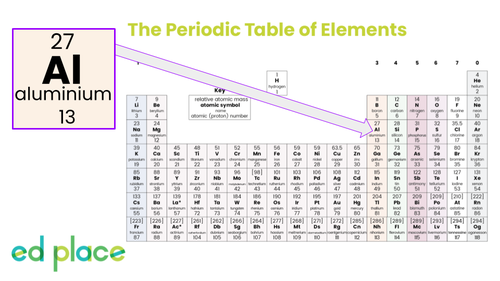
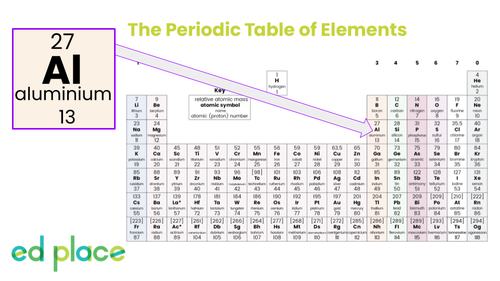
Aluminium
Atomic Number: 13
Symbol: Al
Symbol: Al
8
New cards
How many neutrons does an aluminium atom have?
14

9
New cards
How many electrons does an aluminium atom have?
13
10
New cards
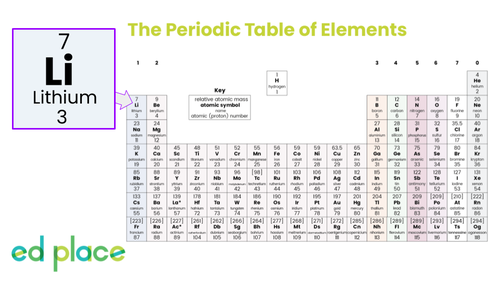
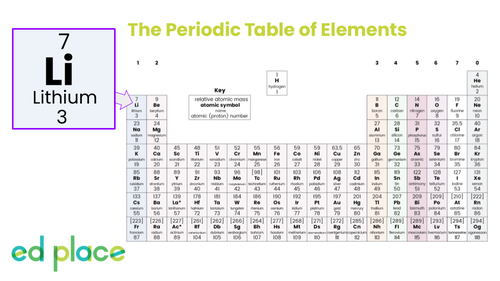
What is the mass number of lithium?
7
11
New cards
How many electrons does a lithium atom contain?
3
12
New cards
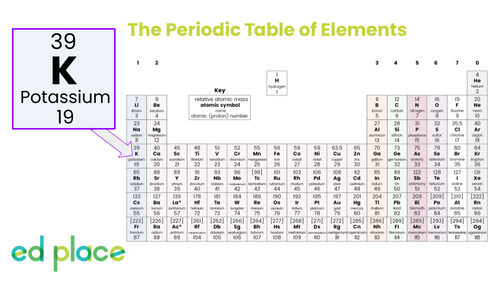
How many protons are there in an atom of potassium?
19
13
New cards
How many neutrons are there in an atom of potassium?
20

14
New cards
Can you draw a sodium atom?
Sodium
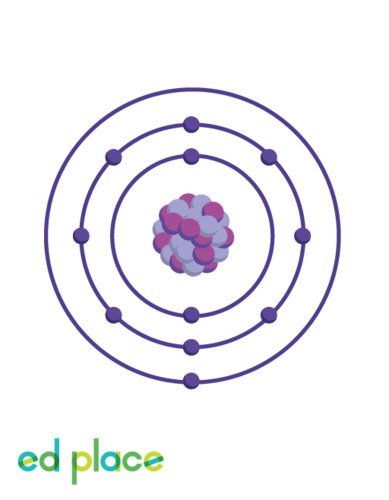
15
New cards
How many electrons does a sodium atom have?
11
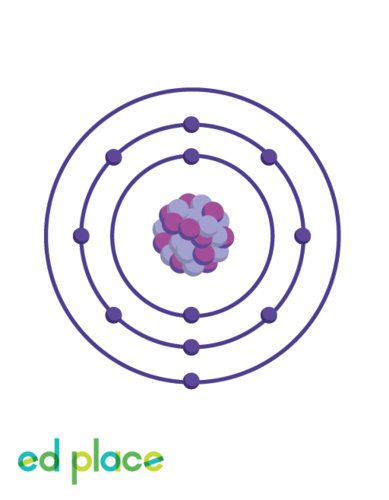
16
New cards
What is the electron structure of a sodium atom?
2.8.1
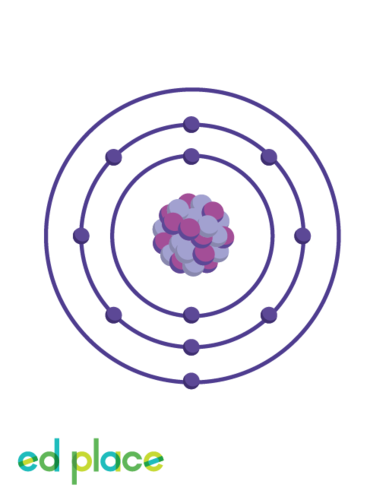
17
New cards
How many electrons are there in an aluminium atom?
13
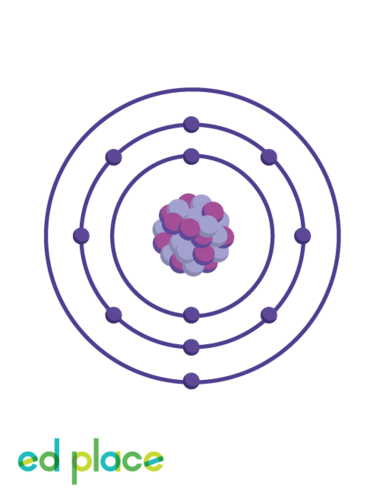
18
New cards
What is the electron structure of aluminium?
2.8.3
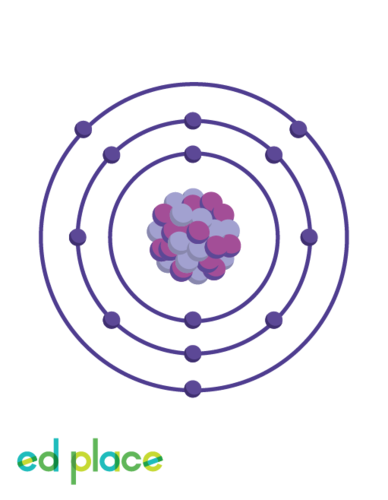
19
New cards
What group can aluminium be found in?
Aluminium is a group 3 element
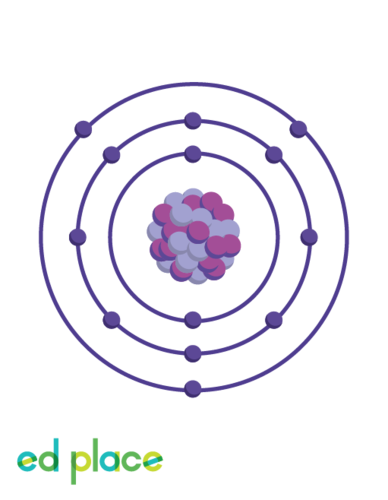
20
New cards
What is the atomic number of aluminium?
13