Lecture 6: Generation of Antibodies
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
B-Cells
B-cells must differentiate into plasma cells to produce antibodies
B-cells themselves do not secrete antibodies
Plasma cells, derived from antigen-specific B-cells, are the true antibody-producing cells
Antibodies are made by the B lymphocyte lineage
"B" stands for Bursa (of Fabricius) — a lymphoid organ in chickens where antibody-producing cells develop
Mammals don’t have a bursa, but the name stuck
Cells originate in the bone marrow then migrate to secondary lymphoid tissues (e.g. lymph nodes, spleen)
Naïve B- and T-cells circulate via the blood and enter lymph nodes through high endothelial venules (HEVs)
→ This ensures they’re in the right place to encounter antigens
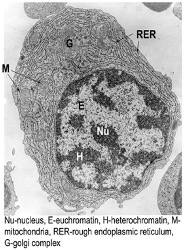
Plasma Cell
Very specific cellular structure
Heterochromatic nuclei as large areas of the DNA are open, as the plasma cells are cells that pump out protein to generate lots of Ab
Have lots of Golgi apparatus and ER to allow them to keep processing proteins
Described as having a cartwheel-shaped nucleus and an unusual cellular make-up due to their specialised function in producing antibody
Development of Antigen-Specific B-cells
B cells develop in the bone marrow, where they:
Rearrange immunoglobulin genes (antigen-independent)
Are supported by specialised stromal cells
They express their rearranged immunoglobulin as membrane-bound IgM molecule, as a B-cell receptor (BCR)
This IgM can also be secreted later as an antibody
If a BCR/ antibody binds strongly to self-antigens, the B cell is eliminated
This prevents autoimmunity and self-reactivity
Upon maturation, B cells also begin to express IgD alongside IgM
Both have the same antigen specificity
Key stages in B-cell development and associated markers
B-cell progenitor develops into a pro-B cell, which expresses CD19
CD19 is a clinical marker used to identify B cells (e.g. in flow cytometry)
Pre-B cells begin to express IgM (via rearranged Igu heavy chain)
This gives rise to an immature B cell with membrane-bound IgM
Matures further into a mature B cell, expressing both:
Membrane IgM
Membrane IgD
Both have the same antigen specificity
B-Cell Fate After Development
Cells leave the bone marrow and move around the body to populate secondary lymphoid organs and re-circulate
When they encounter their specific antigen in the lymph nodes (in the cortex), they proliferate and eventually differentiate into plasma cells and long-lived memory B-cells (respond more quickly upon secondary challenge/infection)
B-Cell Development: Fate of Cells
B-cells, following specific antigen recognition, undergo clonal proliferation
It differentiates into:
Plasma cells that produce antibodies
Memory B-cells for long-term immunity
All resulting cells retain the same antigen specificity
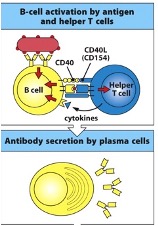
B-Cell Activation
Require 2 signals for activation:
Signal 1: Antigen recognition via membrane-bound Ig (IgM/IgD)
Signal 2: Usually provided by CD4+ T cells (T cell-dependent activation)
T-Cell Independent Stimulation
Certain antigens (e.g. bacterial polysaccharides) can directly activate B cells without T cell help → deliver strong enough antigens to stimulate B-Cels without T-cells
Triggered by repetitive antigen structures on the pathogen surface → BCR activated as it sees ‘lots of the antigen close together’
Results in low-affinity antibodies, as there is no affinity maturation
T-Cell Dependent Activation
T-cell expresses CD40 ligand on its surface, which can ligate CD40. Of the B-cell to help it become activated
T-helper cells can also produce cytokines e.g. IL-2 which can assist in B-cell proliferation and differentiation
Conformational antigen recognition by B-Cell
Where Do B-Cells Meet Antigen
B-cells meet antigens in the lymph node
B-cells localised in the cortex (T-cells in the paracortex), in the absence of an antigen-specific response
B-cells are in close proximity to subcapsular macrophages in the subcapsular sinus
B-cells near antigen-presenting cells
Antigen Recognition in the Lymph Node → Role of Macrophages
Antigen entering lymph nodes or spleen are collected by specialised macrophages in the marginal zone or subcapsular sinus
These macrophages preserve the conformational epitopes of antigens so that:
B cells in the cortex can directly recognise them via BCRs (no breakdown needed)
In contrast, T cells recognise peptide antigens presented by MHC II on dendritic cells
Physical conformational antigens are often repetitive in structure, leading to simultaneous engagement (cross-linking) of many BCRs → recognition by many B-Cells at the same time
This cross-linking is crucial for B-cell activation
Macrophages help present antigens in a way that enables this cross-linking - need more that one BCR (Ig) on the surface that must be stimulated or cross linked
Marginal Zone Macrophages in the Lymph Node
Located in the cortex close to where the B-cells are present
Actions Following B-Cell Activation
Activated B cells move toward the border of the cortex and paracortex
At the same time:
Dendritic cells (DCs) present peptide antigens via MHC II to CD4+ T cells in the paracortex, activating them
T cells and B cells that recognise the same antigen are activated simultaneously but via different forms (conformational vs peptide)
Eventually, activated B cells and CD4+ T cells meet at the cortex-paracortex border for further interaction and differentiation
📍 Reminder: The paracortex is where CD4+ T cells are activated
Germinal Centre Formation
Activated B and T cells first form primary foci in the medullary cords of the lymph node
These are areas of initial proliferation
From the primary foci, some activated B cells migrate back into the cortex, entering primary follicles
This leads to the formation of germinal centres within the follicles, where B cells undergo further maturation
How Do B-Cells Interact With T-Cells
B cell binds antigen (e.g. from a macrophage), internalises the antigen, and processes it to become activated
B cell then expresses MHC Class II with the processed peptide on its surface
B cells are one of the few cells that can produce MHC Class II!
Meanwhile, T cells activated by dendritic cells recognise the same MHCII–peptide complex on the B cell
This allows B cell–T cell interaction, enabling further B cell activation and support
T-Cell Co-Stimulation
T-cells help the B-cells to drive the process forward and enhance the proliferation process
B-cell bound to a viral coat protein and will then internalise and degrade the viral coat protein
Peptide from this viral particle will be expressed by MHCII on the B-cell surface that interacts with a T-follicular helper cell, which can help to activate the B-cell by giving co-stimulation to the B-cell via CD40 ligand
T-Follicular Helper Cell (CD4+)
If these cells have the right specificity, they deliver the second signal to B cells by recognising the peptide–MHCII complex presented by the B cell
This Tfh–B cell interaction assists with antibody production
Ensures only T cells with matching antigen specificity provide help to B cells, maintaining specificity in the immune response
T-Helper Cells Role in B-Cell Differentiation
Helper T cells adhere to B cells and engage in CD40–CD40L interactions, initiating synthesis of IL-4, a cytokine important for B cell differentiation
After antigen-specific recognition, the T cell cytoskeleton rearranges, directing the secretory apparatus toward the site of B and T cell interaction
This can be visualised using Talin staining
IL-4 is selectively and directionally released toward the B cell, creating a high local concentration to ensure effective signalling
Clonal Expansion and Differentiation of B-Cell
Occurs in response to a series of interactions of the antigen-specific B-cell.
It involves the following signals:
co-stimulatory molecules eg CD154 (CD40L) on the T cell and CD40 on the B cell
Cytokines from the T follicular helper cell
The results of these interactions in B-cell proliferation
Consequences of B-Cell Proliferative Events
Some activated B cells become plasma cells/plasmablasts, rapidly secreting IgM
IgM is the first antibody produced against an antigen
It has low affinity and hasn’t undergone affinity maturation
B cell proliferation, affinity maturation, and class switching occur after activation
Class switching allows plasma cells to produce other antibody types, e.g. IgG
How do activated B cells and Tfh cells contribute to affinity maturation in the lymph node?
After 4–7 days, some activated B cells and T follicular helper (Tfh) cells move to the cortex of the lymph node
They enter primary follicles, which are specialised regions
Primary follicles contain Follicular Dendritic Cells (FDCs) – a unique type of antigen-presenting cell important for affinity maturation
Folicular Dendritic Cells
These cells, not derived from haematopoietic origin, form a network throughout the primary follicle
They are specially designated to hold antigen/ antibody complexes on their surfaces in small nodules - iccosomes
The antibody intially comes fro the plasma cells in the extra follicular region of the cortex
Role of FDCs and Germinal Centres in Antibody Response
FDCs in primary follicles hold antigens for extended periods to provide a sustained source of antigens for B cells.
This supports the next stage of the antibody response: the formation of Germinal Centres.
FDCs display antigens to B cells over time, helping maintain stimulation and supporting affinity maturation.
Germinal Centres
Are the site of affinity maturation, where antibodies go from low to high affinity.
They have a defined structure:
Centroblasts: proliferating B cells that express IgM
Centrocytes: non-dividing B cells that undergo selection
Affinity Maturation
Activated B cells entering primary follicles down-regulate their Ig membrane receptors and proliferate (extensively) into centroblasts.
During proliferation, B cells undergo affinity maturation, leading to high-affinity antibody production.
As the centroblasts divide, affinity maturation involves hypermutation of heavy (H) and light (L) chains of the Ig molecule, randomly altering the structure of hypervariable regions.
This process results in antibodies with either higher or lower affinity for the antigen.
What happens during the selection of high-affinity B cells in the germinal centre after affinity maturation?
Centroblasts stop dividing and re-express surface Ig, becoming centrocytes.
They compete for binding to antigens displayed by FDCs in the follicle.
If centrocytes bind antigen with high enough affinity, they receive a survival signal; otherwise, they undergo apoptosis.
Only B cells secreting high-affinity antibodies survive; low-affinity antibodies are still produced in small amounts.
As antigen levels decrease with the progressive immune response, only B cells with higher affinity antibodies are selected.
Affinity maturation can increase antibody affinity by 10,000 to 100,000 fold!
a small amount of low affinity Abs are still produced
Fate of Centrocytes After Selection in the Germinal Centres
Surviving centrocytes interact with activated T helper (Th) cells again.
They differentiate into:
Plasma cells – secrete large amounts of high-affinity antibody.
Memory B cells – provide long-term protection during secondary infection.
Structure of The Germinal Centre
Dark zone – contains proliferating centroblasts.
Basal light zone – contains selected centrocytes and Follicular Dendritic Cells (FDCs).
Apical light/ Mantale zone – site of plasma cell and memory B cell differentiation.
Class Switching
Occurs during the centroblast.centrocytes stage of B-cell differentiation process
The B cell is able to change its heavy chain constant region from µ (IgM) to γ (IgG) or α (IgA) or ε (IgE) whilst keeping the same heavy chain variable and light chain (the antigen binding parts)
Changes the ‘section’ that binds to a cell
Allows the generation of Abs with the same affinity but is a different class
Control of Class Switching
It is mediated by CD4 T helper cells and cytokines
Without CD40/CD40L only make IgM
Different cytokines induce the production of different antibody classes e.g. IL-4, produced in response to allergens or worm infections induces IgE – effective in these defences
N.B. cytokines also influence how much antibody is made
Combinations of Cytokines That Can Promote Class Switching
Sub-combinations of cytokines that are effective in inducing Ab classes
IL21 + IL4 induce IgG1
IL21 Induces IgG3
IL13 gamma induces IgG3
IL10, IL21 +TGFb induce IgA
IL4 and IL13 induce IgE
INF is effective at inducing
Plasma Cells and Memory B-Cells
Cells formed in the final stage of differentiation
Plasma cells secrete large amounts of antibodies (>2000 Ab molecules per second!)
Memory B cells can survive for long periods – they have undergone class switch and affinity maturation – so when they see antigen for a 2nd time, they can respond with a very quick and efficient response
Formation of Memory B-cells
Occurs in the apical light zone of the germinal centre.
When a B cell interacts with a CD4+ T follicular helper (Tfh) cell,
CD154 (on Tfh) binds CD40 (on B cell).
This interaction drives the B cell to become a memory B cell.
These memory B cells can then exit the lymph node and enter circulation.
Why Do Some B-Cells Become Plasma Cells and Some Become Memory Cells
We do not fully understand
It’s suggested that in the absence of CD154–CD40 interaction, the default pathway is plasma cell differentiation.
Plasma Cells can be:
short-lived (remain in lymph nodes/spleen, secrete Ab for a few weeks).
long-lived (migrate to the bone marrow, secrete Ab for months).
The bone marrow is the main source of long-term antibody production — lots of Ab found in the body is derived from the long lived plasma cells → provides protection against invading pathogens.
Process of Germinal Centre Formation
Occurs 4-14 days after antigen encounter
1. Antibody Class Switch
Cell surface IgM or IgD changes to IgG, IgA or IgE
2. Affinity Maturation of Antibody
Select for antibody with high affinity
3. Differentiation of B cells in memory cells
have undergone class switch and affinity maturation but not differentiated into plasma cells
4. Differentiation of B cells into Plasma Cells