Nucleophilic Substitution and Elimination Reactions of Alkyl Halides
1/28
Earn XP
Description and Tags
These flashcards cover key concepts related to nucleophilic substitution and elimination reactions of alkyl halides, including reaction mechanisms, solvent types, and nucleophile classifications.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Nucleophilic Substitution (SN2)
A bimolecular reaction where the nucleophile attacks the substrate, displacing the leaving group in a single concerted step.
Nucleophilic Substitution (SN1)
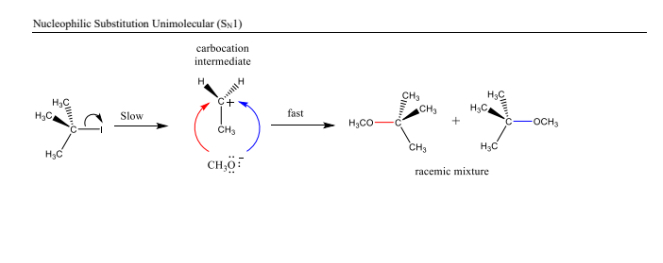
A unimolecular reaction involving the formation of a carbocation intermediate, followed by the nucleophile attack in a separate step.
Elimination Reactions (E2)
A bimolecular elimination reaction that occurs in one step, removing a leaving group and forming a double bond.
Elimination Reactions (E1)
A unimolecular elimination reaction involving the formation of a carbocation intermediate followed by deprotonation.
Zaitsev Rule
The rule stating that the most highly substituted alkene predominates in elimination reactions.
Hofmann Rule
The rule stating that using a sterically hindered base leads to the formation of the least substituted alkene.
Leaving Groups
Species that can depart with a pair of electrons, with weak bases being the best leaving groups.
Polar Aprotic Solvents
Solvents that cannot form hydrogen bonds and have no hydrogen atoms connected to electronegative atoms.
Polar Protic Solvents
Solvents that can form hydrogen bonds because they contain hydrogen atoms connected to electronegative atoms.
Non-Polar Solvents
Solvents that lack partial charges and have little to no dipole moment due to similar electronegativity of their atoms.
Strong Nucleophiles
Nucleophiles that are strong bases; include species like OH⁻, RO⁻, and NH₂⁻.
Weak Nucleophiles
Substances that have little to no reaction with electrophiles, such as water and alcohols.
Carbocation Stability
The stability of carbocations increases from primary to tertiary due to hyperconjugation and inductive effects.
Transition State
A high-energy state during a reaction representing the point of maximum energy along the reaction pathway.
Inversion of Configuration
The change in stereochemistry during SN2 reactions, where the configuration of the chiral center is reversed.
Steric Hindrance
A factor that reduces the ability of a nucleophile to attack a substrate, usually due to bulkiness of the nucleophile.
Alkyl Halides
Organic compounds containing a halogen atom bonded to an alkyl group.
Nucleophilic Substitution (SN2) Reaction Steps
- A strong nucleophile attacks the electrophile. 2. The leaving group departs simultaneously in a single concerted step. Recognized by: A primary substrate and strong nucleophile.
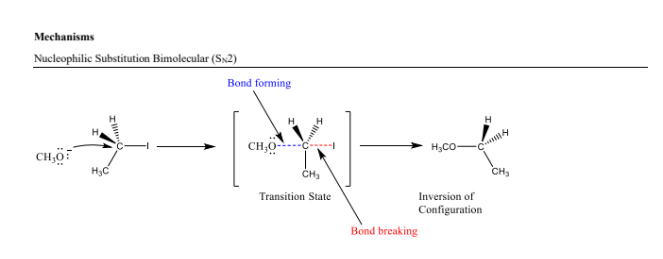
Nucleophilic Substitution (SN1) Reaction Steps
- The leaving group departs first, forming a carbocation. 2. The nucleophile attacks the carbocation. Recognized by: A tertiary substrate or a stable carbocation.
Elimination Reactions (E2) Reaction Steps
- A strong base abstracts a proton, forming a double bond while the leaving group departs. Recognized by: Strong bulky base and presence of a good leaving group.
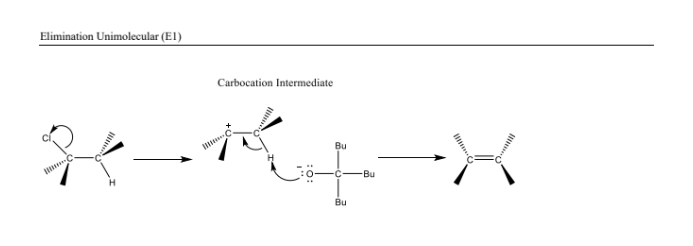
Elimination Reactions (E1) Reaction Steps
- The leaving group departs first to form a carbocation. 2. A base abstracts a proton to form the double bond. Recognized by: A stable carbocation and weak base.

Formulas for Nucleophilic Reactions
For SN2: $A + B \rightarrow C$ (where A is substrate, B is nucleophile, C is product). For SN1: $A \rightarrow B + C$ (A is substrate, B is carbocation, C is nucleophile).
Recognizing SN2 vs SN1
- SN2: Strong nucleophile, primary substrates, spatial arrangement matters.
- SN1: Stable carbocation formations, tertiary substrates, involves rearrangement.
Recognizing E2 vs E1
- E2: Strong, bulky base is required, simultaneous removal of H and leaving group.
- E1: Carbocation stability, commonly found in secondary or tertiary substrates
What are the common characteristics of nucleophiles?
Nucleophiles are species that donate an electron pair to form a chemical bond with an electrophile, often characterized by high electron density.
What is a carbocation and its significance in nucleophilic substitution?
A carbocation is a positively charged carbon atom, and it is significant because it serves as an intermediate in SN1 reactions and affects the reaction pathway.
What factors affect the stability of carbocations?
Carbocation stability is influenced by factors like the number of alkyl substituents and resonance stabilization, with tertiary carbocations being the most stable.
What is the effect of solvent type on nucleophilic reactions?
The type of solvent can affect the nucleophilicity of reactants, with polar protic solvents stabilizing ions and polar aprotic solvents enhancing nucleophilicity.
How do reaction mechanisms differ between SN2 and SN1?
SN2 involves a concerted one-step mechanism, while SN1 involves a two-step mechanism with carbocation formation followed by nucleophile attack