VSEPR Theory
1/20
Earn XP
Description and Tags
Chem Unit 2 Y11 ATAR
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
what does VSEPR Theory stand for?
Valence Shell Electron Pair Repulsion (VSEPR) theory
VSEPR Theory
Negatively charges electron pairs in the outer shell of an atom repel each other.
These electron pairs are arranged far away from each other as possible.
Lone pairs of electrons influence a molecules shaped but are not considered a part of the shape
Shape of molecules: tetrahedral
No. atoms | No. of bonds | No. lone pairs | Shape |
5 | 4 | 0 | tetrahedral |
Shape of molecule: pyramidal
No. atoms | No. of bonds | No. lone pairs | Shape |
4 | 3 | 1 | pyramidal |
Shape of molecule: Trigonal planar
No. atoms | No. of bonds | No. lone pairs | Shape |
4 | 4 | 0 | Trigonal planar |
Shape of molecule: V-shaped (bent)
No. atoms | No. of bonds | No. lone pairs | Shape |
3 | 2 | 2 | V-shaped (bent) |
Shape of molecule: linear
No. atoms | No. of bonds | No. lone pairs | Shape |
2 | 1 | 3 | Linear |
3 | 4 | 1 | Linear |
3 | 2 | 0 |
Double bonds, triple bonds and valence shell electron pair repulsion theory
double and triple bonds are considered to be just one 'cloud' of electrons is treated in the same way as a single bond.
if a central atom has two single bonds and one double bond, then the three sets of bonds will repel each other to get maximum separation.
results in a molecular shape known as trigonal planar because the atoms form a triangle in one plane. An example of this structure is the methanal molecule (CH,O)
Trigonal planar molecules have bond angles of 120°.
If the central atom has two double bonds, then the two double bonds repel each other. This results in a linear molecule like carbon dioxide (CO2).
single bond and a triple bond, as in hydrogen cyanide (HCN), then linear
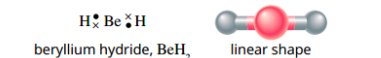
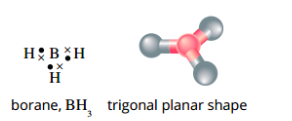
Molecules that do not obey the octet rule
In borane (BH), the central boron atom is bonded to three hydrogen atoms, but has no lone pairs. There are only three pairs of electrons in this molecule.form a trigonal planar molecule
VSEPR theory predicts that the three electron pairs will move as far away from each other as possible into a trigonal planar shape.
beryllium hydride (BeH2), the central beryllium atom forms single bonds with two hydrogen atoms, but has no lone pairs of electrons. The two single bonds repel each other to form a linear molecule
lone pairs
In water molecules, the oxygen atom has a stable octet made up of two lone pairs and two single bonds.
The four electron pairs repel each other to form a tetrahedral arrangement.
two hydrogen atoms to form a V-shape or bent arrangement with the oxygen atom
With two lone pairs in the molecule, the two single covalent bonds are pushed closer together. In this case, the bond angle around the central atom is also less than 109.5°.
v shaped/bent
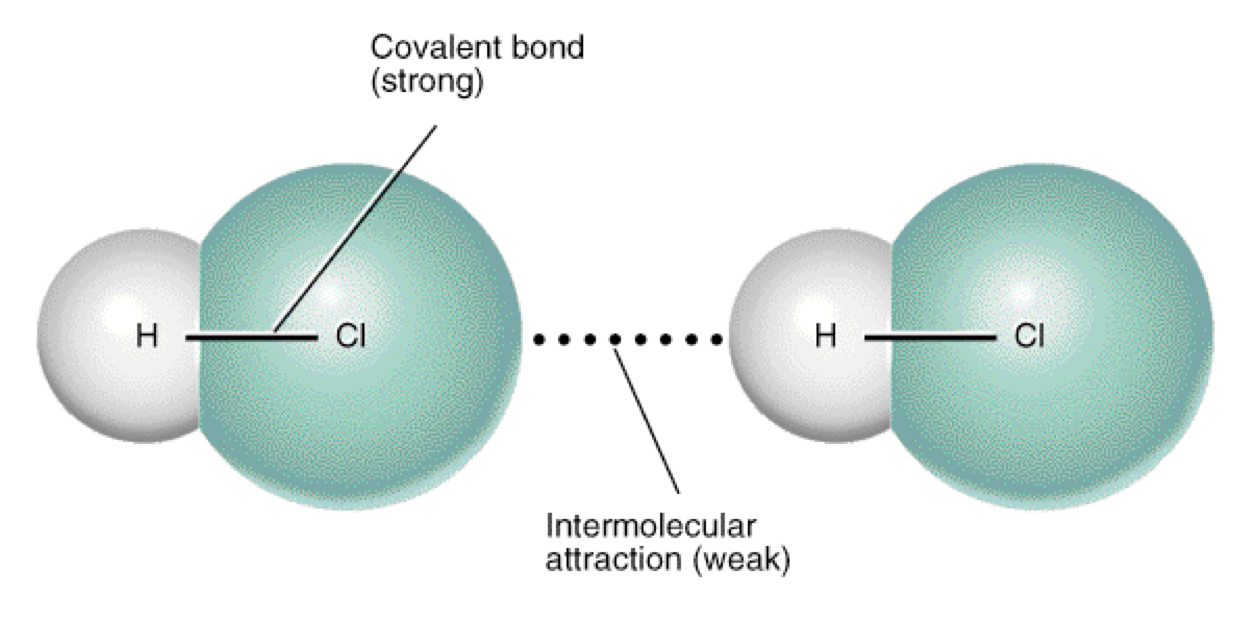
Intermolecular force
determine physical properties such as vapour pressure, melting point, boiling point, and solubility.
physical properties: vapour pressure
•Liquid is put into a closed container, some of the particles escape from the surface liquid. These particles form a gas or vapour that exerts a pressure on the container due to their collisions with the container wall.
physical properties: melting point
•The melting point is the temperature at which a solid changes into a liquid. The same as the freezing point of the liquid.
physical properties: boiling point
•The boiling point of a substance is the temperature at which the vapour pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapour.
liquids changing to gases
•When liquids change to gases, the intermolecular bonds are broken. For example, when liquid water changes to gaseous water, the intermolecular bonds holding the water molecules are broken, not the covalent bonds between the hydrogen and oxygen atoms.
imf of the different states
Gases: the average kinetic energy of the gas molecules is much larger than the average energy of the attraction between them.
Liquids: the intermolecular attractive forces are strong enough to hold the molecules close together, but without much order.
Solids: the intermolecular attractive forces are strong enough to lock molecules in place (high order).
strength of imfs
• Both the shape of a molecule and the nature of its covalent bonds must be taken into account when predicting the strength
imf is an electrostatic force
•Examples: cation+anions in ionic lattice; attraction of shared electrons and nuclei in covalent bonds – INTRAmolecular forces
•They are based on the similarity or differences in electronegativity.
polar
has a net dipole, where there is a slight negative at one end of the molecule, and a slight positive at the other. δ+ is the positive dipole, and δ- is the negative.
non-polar
When the intramolecular bonds in an atom are not polar, or if the molecule is symmetrical, and therefore has vectors that cancel each othe
strength of bonding:imf
•The strengths of intermolecular forces are generally weaker than either ionic or covalent bonds.