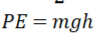Physics Chapter 9
5.0(1)
5.0(1)
Card Sorting
1/30
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
1
New cards
Kinetic Energy being added to the particles of a substance
is a direct cause of the substance’s temperature increase
2
New cards
Temperature is proportional to
the kinetic energy of atoms and molecules
3
New cards
Vibrational kinetic energy is the form of kinetic energy
that occurs within a molecule when the bonds are bent or stretched
4
New cards
Internal energy is
the energy associated with atomic motion
5
New cards
Energy transferred as heat occurs between
two bodies in thermal contact when they differ in temperature
6
New cards
Energy transferred as heat is always
directed from an object at a high temperature to an object at a low temperature
7
New cards
If energy is transferred from a table to a block of ice moving across the table
that means the ice is cooler than the table
8
New cards
Energy transferred as heat between two objects depends on
the difference in temperature of the two objects
9
New cards
1. A calorimeter is used to determine the
2. If the specific heat capacity of water is known
1. the specific heat capacity of a test metal
2. you must still measure the metal mass, water mass, and initial and final temperatures of the metal and water
10
New cards
*Understand a phase change graph*
11
New cards
Internal energy is the energy due to
both the random motions of the substance’s particles and the potential energy due to the bonds between those particles
12
New cards
Rotational kinetic energy is the type of kinetic energy
associated with a molecule spinning about its center of mass
13
New cards
Two systems are in thermal equilibrium when
they have the same temperature
14
New cards
Thermal Expansion:
As the temperature increases, the volume of the substance increases
15
New cards
The Celsius and Kelvin temperature scales are similar because
the difference of one degree is the same for both scales
16
New cards
Convection is
the process by which energy is transferred by the motion of hot and cold matter
17
New cards
Specific heat capacity is
the quantity of energy needed to raise the temperature of a unit mass of a substance by 1 degree C
18
New cards
The properties of a substance needed to determine the amount of energy transferred as heat to or from a substance are
temperature change, specific heat capacity, and mass
19
New cards
In a heating curve
a line with a positive slope indicates the increase in the substance’s temperature with added energy
20
New cards
Temperature measures
the average kinetic energy of particles in a substance
21
New cards
If two objects are in thermal equilibrium
their temperatures are the same
22
New cards
Materials that easily transfer energy heat are called
thermal conductors
23
New cards
If two objects are in thermal equilibrium the
particles in each object have the same average kinetic energy
24
New cards
A phase change is a
physical change of a substance from one state (solid, liquid, or gas) to another state at constant temperature and pressure
25
New cards
Celsius-Fahrenheit Temperature Conversion
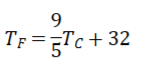
26
New cards
Celsius-Kelvin Temperature Conversion
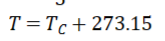
27
New cards
Conservation of Energy
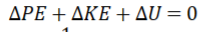
28
New cards
Specific Heat Capacity
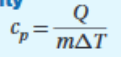
29
New cards
Energy conservation can be used to calculate the specific heat \n capacity
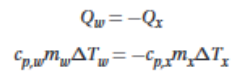
30
New cards
Kinetic Energy
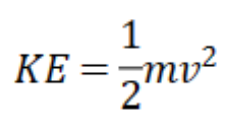
31
New cards
Potential Energy