Unit 2 IB CHEM
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
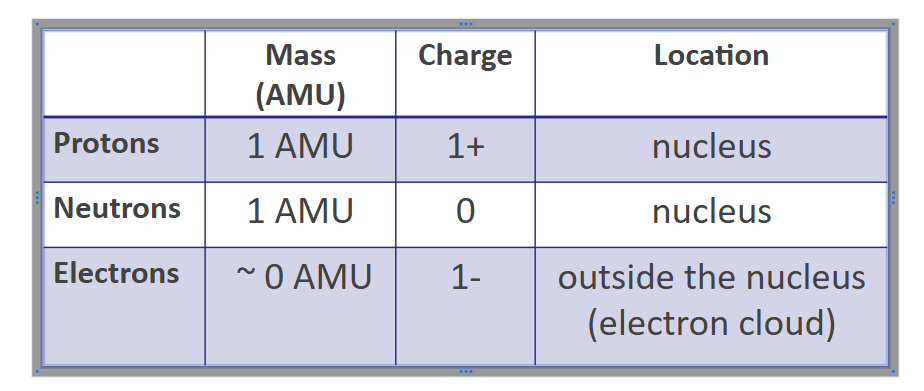
AMU & Location of Electron, Neutron, and Protons
Isotopes can be written in two ways:
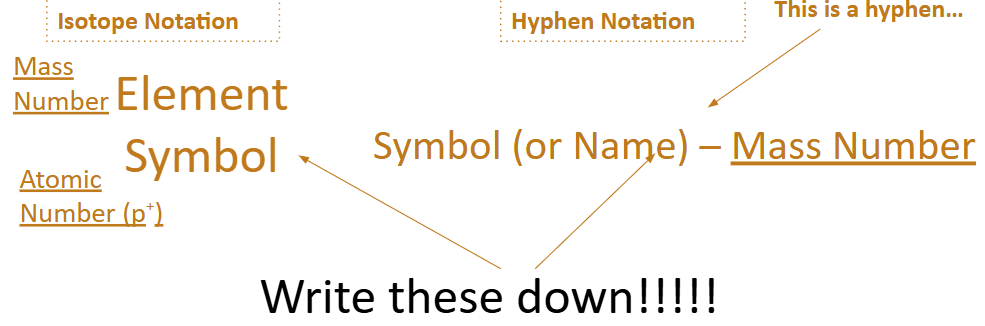
Isotopes
Isotopes = atoms of the same element with a different number of neutrons and mass
Properties of Isotopes
Chemical Properties of isotopes of the same element are the same
Because chemical behavior is associated with electrons not neutrons
Physical Properties of isotopes of the same element are different
Physical properties are based on mass
Densities, MP, BP, etc…will be different
Some isotopes are radioisotopes (radioactive)
Mass Number
The mass number is the number of protons and neutrons in the nucleus.
Mass Number is NOT the actual mass of an atom or element
Average Atomic Mass
Average atomic mass is an AVERAGE of ALL of that element in the universe.
Atomic Mass is expressed in Atomic Mass Units → amu
The periodic table lists average atomic mass based on mass and percent (%) abundance of each naturally occurring isotope of an element.
Average atomic mass is a weightedaverage
Abundance
Abundance refers to the amount of each isotope
The average atomic mass of an element is going to be closest to the most abundant isotope
Average Atomic Mass Formula
(Mass x Abundance % ) + (Mass x Abundance % ) + …
100 100
Valence electrons
The valence electrons are the outermost electrons (the highest energy level).
Valence electrons determine chemical properties.
Every element wants to have a full octet = 8 Valence e-
Exceptions:
H: Full with 2 valence e-
B: Full with 6 valence e-
Elements will gain or lose valence electrons to get a full octet, this is how ions are formed
Periods
There are 7 periods
Elements in the same period have the same number of electron shells/energy levels
The Horizontal rows
Groups/Families
The vertical columns of the periodic table are called GROUPS or FAMILIES.
The elements in any group of the periodic table have similar physical and chemical properties.
Identifying Electrons in an Atom
4 Unique Quantum Numbers are used to identify each electron in an atom
Principal Quantum Number- Principal energy level (n)
Second Quantum Number- Sublevel (l)
Magnetic Quantum Number- Orbital (ml)
Spin Quantum Number- Spin (ms)
Principal Quantum Number
Principal Energy Level (Energy Level)
positive integer
n = 1, 2, 3, 4, ...
larger numbers are farther away from the nucleus - higher energy level
Energy level 1 = max 2 electrons
Energy level 2 = max 8 electrons
Energy level 3 = max 18 electrons
Energy level 4 = max 32 electrons
Second Quantum Number
Sublevel - type or Shape of Atomic Orbitals
You can remember this with the saying:
Some People Do Fine
Principal Energy Level | Sub-levels |
1 | s |
2 | s, p |
3 | s, p, d |
4 | s, p, d, f |
Shape of S Orbital (Must know how to draw)
Shape of P orbital (Must know how to draw)
Shape of d orbital and f orbital (not required to draw)
Magnetic Quantum Number
Represents the number of orbitals in the sublevel (which orbital are they in?)
Each orbital can hold up to 2 electrons
Sub-level | Number of orbitals | Number of electrons |
s | 1 | 2 |
p | 3 | 6 |
d | 5 | 10 |
f | 7 | 14 |
Principal Energy Level | Sub-level | # of orbitals in sub-level | # of electrons in Energy Level | |
1 | s | 1 | 2 | |
2 | s | 1 | 2 |
|
p | 3 | 6 | ||
3 | s | 1 |
|
|
p | 3 | |||
| ||||
d | 5 | |||
| ||||
4 | s | 1 |
|
|
p | 3 |
| ||
d | 5 |
| ||
f | 7 |
| ||
Not a flashcard
Principal Energy Level | Sub-level | # of orbitals in sub-level | # of electrons in Energy Level | |
1 | s | 1 | 2 | |
2 | s | 1 | 2 |
|
p | 3 | 6 | ||
3 | s | 1 |
|
|
p | 3 | |||
| ||||
d | 5 | |||
| ||||
4 | s | 1 |
|
|
p | 3 |
| ||
d | 5 |
| ||
f | 7 |
| ||
Not a flashcard
Spin Quantum Number
Orientation of Electron (Spin)
Each orbital can only hold 2 electrons
Electron spin in the same orbital is opposite
ms = + ½ or - ½
Rule #1: Aufbau Principle
Start filling electrons at the lowest energy level. One electron at a time
Rule #2: Pauli Exclusion Principle
A maximum of 2 electrons can be in one orbital. They must have opposite spins.
Rules #3: Hund’s Rule
Electrons fill each orbital in a sublevel before they start pairing up
Noble Gas Configuration
Find the noble gas that is one row above your element.
Write that noble gases symbol in [ ].
Then write the remaining sublevels that are filled by your element.
Valence Electrons
Valence electrons are electrons held in highest energy level – typically the last s or s and p sub-levels
Ground State and Excited State
When the electrons in an atom become excited by absorbing energy from their surroundings, they jump to new higher energy levels
Electrons in the lowest energy level possible are in the ground state.
Electrons in ANY higher level are in an excited state.
Relation between length of a wave and its energy
The length of the wave is inversely related to its energy
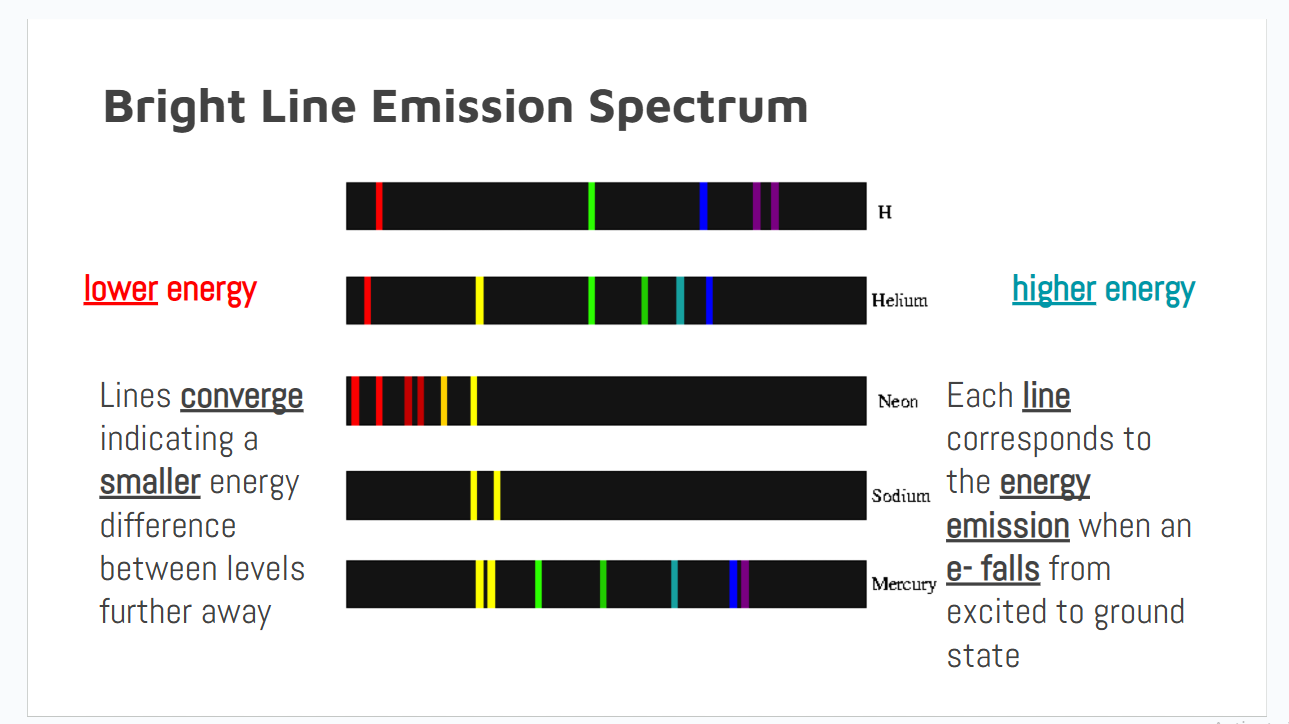
Not a flashcard
Not a flashcard
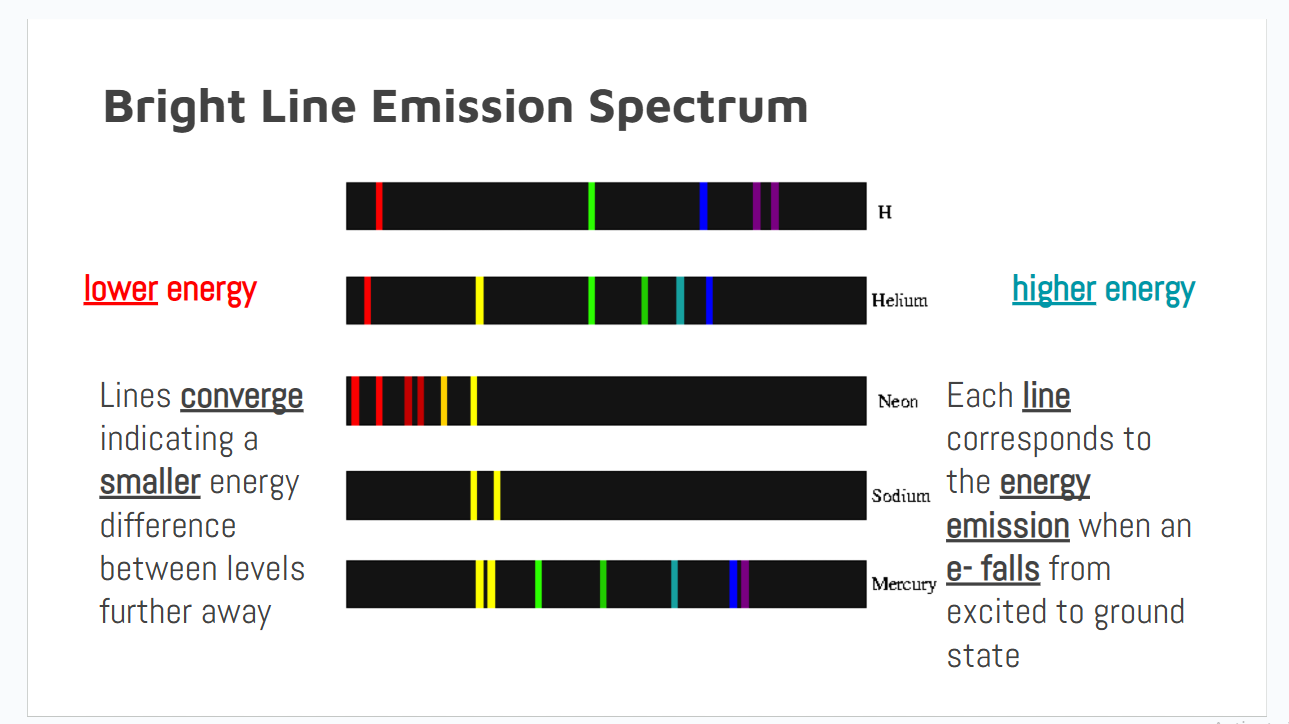
Return to the Ground State
When electrons fall back, a specific amount of energy is emitted based on the distance between electron orbitals.
Orbitals further from the nucleus are closer together.
Return to the Ground State - 1st energy level
When electrons return to the 1st level, the lines appear in the ultraviolet region (Lyman series) since it is the largest energy change
Return to the Ground State - 2nd energy level
When electrons return to the 2nd level the lines appear in the visible region (Balmer series).
Return to the Ground State - 3rd energy level
When electrons return to the 3rd level, the first series of lines in the infrared region (Paschen series) is produced.
Data that line spectra’s give
1. Electrons exist in distinct energy levels because each line represents the energy emitted as an electron falls from excited to ground state
2. Energy differences are smaller between levels that are further from the nucleus because lines are closer together on the higher energy end of the spectrum
Alkali Metals
Properties
Metallic properties
Soft
Can be cut with knife
Reactivity increase down the group
highly reactive with O2, H2O
produce alkali (base solns)
Electron Configuration Xs1
Lose 1e- → Noble Gas
Alkaline Earth Metals
Properties
harder, denser, stronger, and higher melting point than alkali metals
react with O2
Reactivity increases down
Electron Configuration Xs2
Lose 2e- → Noble Gas
Transition Metals
Properties
metals, harder, denser, higher melting point (except Hg)
not as reactive as groups 1&2
Inner transition elements
Lanthanides 58-71
Actinides 90-103
Halogens
Combine with metals to form salts
NaCl
most reactive nonmetals
Reactivity decreases down
Electron Configuration
Xs2 Xp5 (gain 1e- → Noble Gas)
Noble Gases
Inert Gases
Unreacted
Already Full Shell ( 8 Valence e-)
Stable
Electron Configuration Xs2 Xp6
except He - 1s2
Other elements want the same configuration
Nuclear charge (trend)
Across – increases
Down – increases
Nuclear charge – the charge in the nucleus or the number of protons
Valence Electrons (trend)
Valence Electrons – electrons in the outermost energy level (s and p orbitals only!)
Across – increase
Down – stays the same
Average Atomic Mass (trend)
Average Atomic Mass – weighted average based on mass and percent abundance of each naturally occurring isotope (Remember there are exceptions to this (Te/I ) but it is generally true)
Across – increases
Down - increases
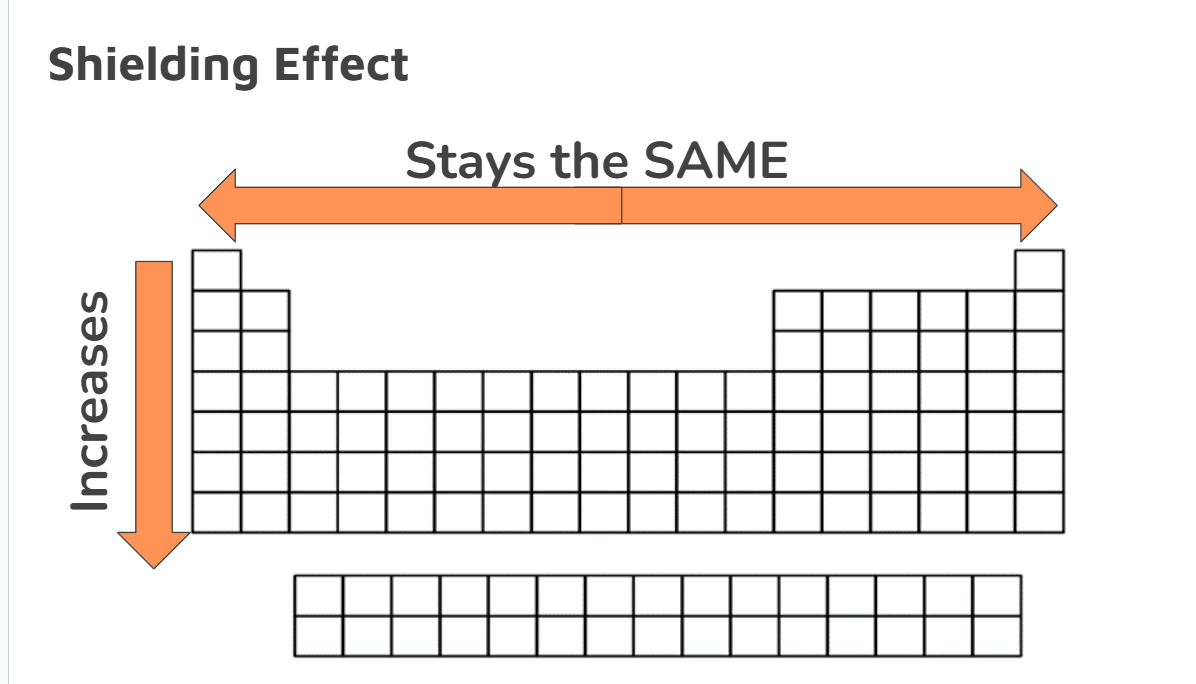
Shielding Effect
The shielding effect is the reduction of attractive force between the nucleus and its valence electrons due to the blocking effect of the shielding electrons
Across the period, stays the same
Electrons are added to valence shell
The number of “inner” electrons”remain the same
Same number of electron shells
Down the group, it increases
Energy level are being added
Inner electrons increase
Atomic size increases
More distance between valence shell and the nucleus
Bigger distance → less attraction = higher shielding effect
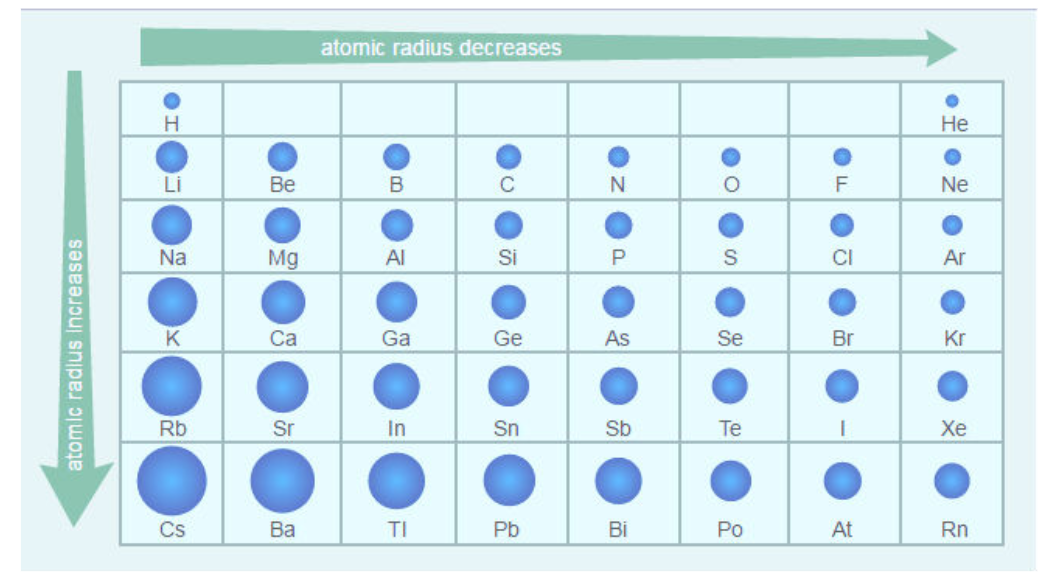
Atomic Radius
Half the distance from center to center of like atoms
Across the period, decreases
The number of protons increases
Down the group, increases
Energy levels are being added, leads to bigger atomic size
Shielding increases
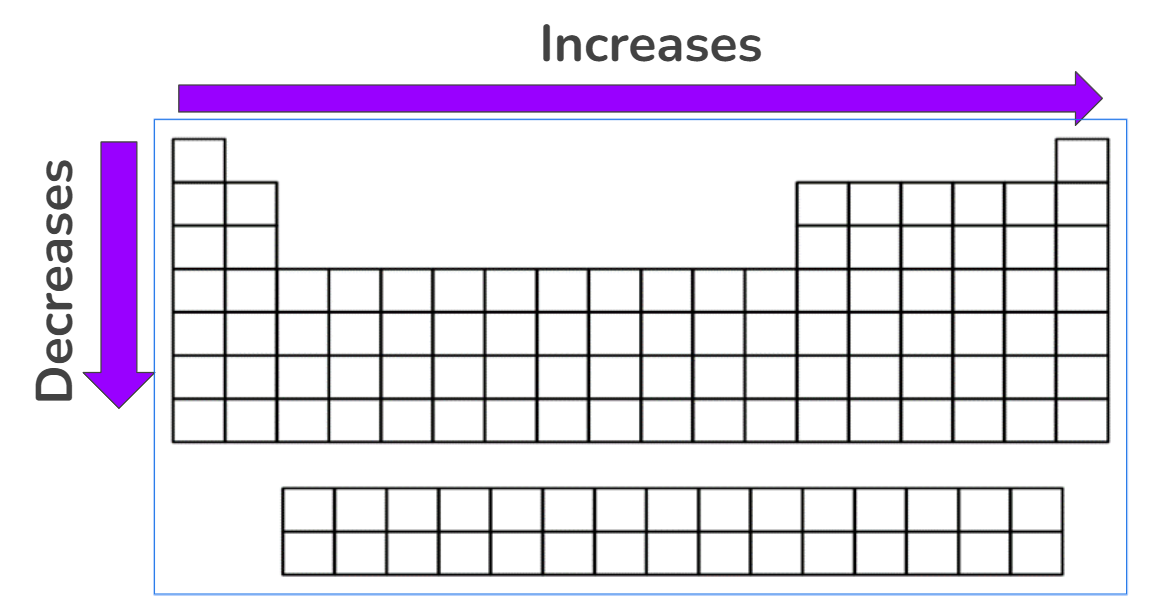
Ionization Energy
The amount of energy needed to remove an electron from an atom
Across the period, increases
Greater the attraction between the valence electrons and the nucleus
Down the group, decreases
Shielding increases, thus atoms get bigger
Electronegativity
The ability for an atom to attract electrons to itself when it is bonded to another atom
Across the period, increases
More protons are being added → Greater attraction for electrons
Down the group, decreases
Increased in shielding, which blocks nuclear attraction
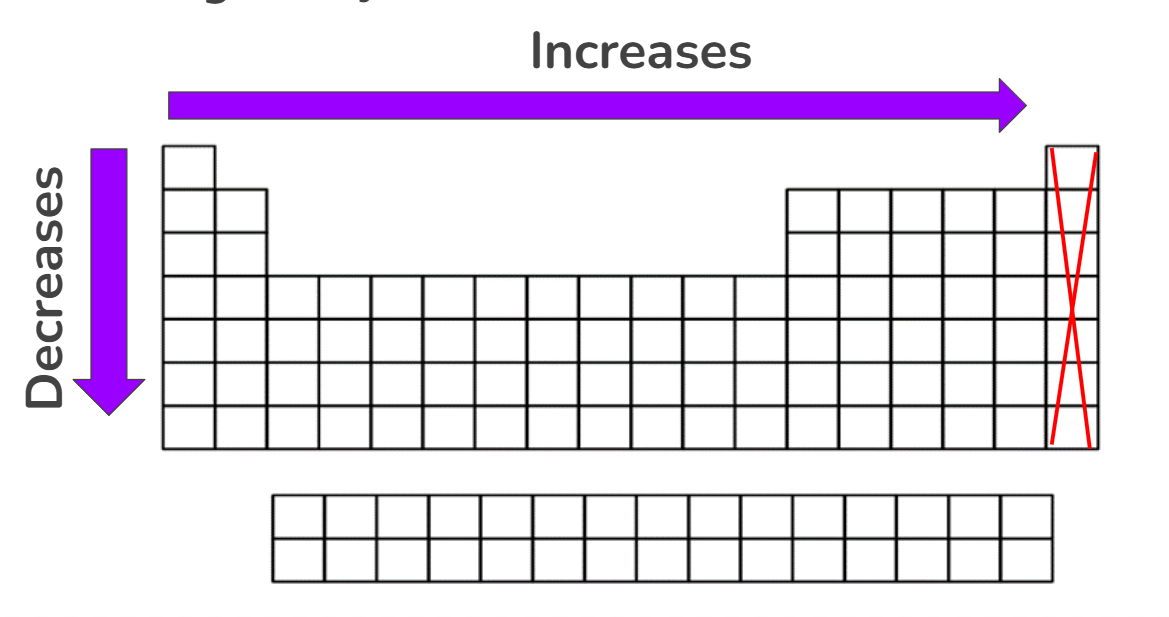
Electronegativity Exceptions
Noble gases have an electronegativity of zero because they are inert or unreactive.
Already have a full valence shell
Octet rule - 8 valence electrons
Reactivity
Reactivity is how readily an element will react with another element.
Metals and Non-metals don’t follow the same trends.
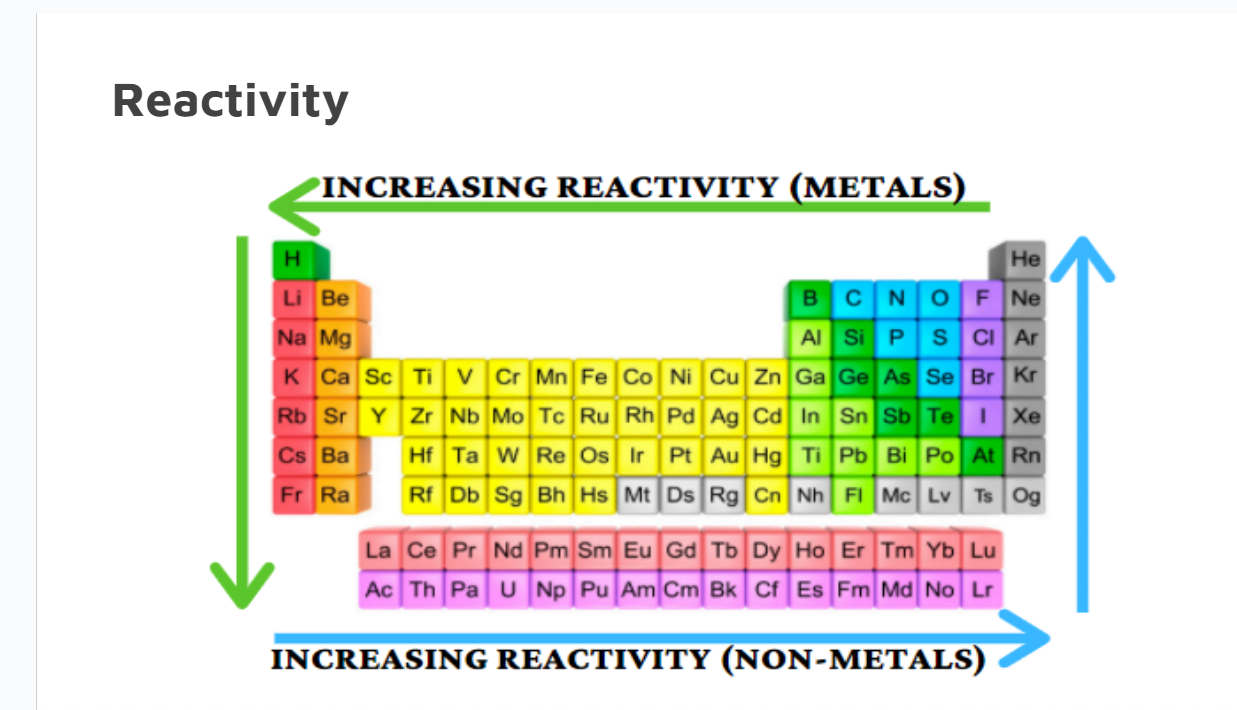
Reactivity in Metals
Across the period, decreases
Nuclear charge increases
Energy levels stay constant, so ionization energy increases.
Down a group, increases
Nuclear charge increases,
Energy levels (shielding effect) increases, so ionization energy decreases.
Reactivity in Nonmetals
Non-metals react by gaining electrons.
They have a high electronegativity , so it's fairly easy for them to gain electrons
Across the period, increases
Energy levels stay constant, so electronegativity increases.
Down the group, decreases
Energy levels (shielding effect) increases, so electronegativity decreases.
Isoelectronic Series
A series of ions/atoms with the same electron configuration (valence isoelectronic)
O2-, F-, Ne, Na+, Mg2+
1s22s22p6
Ionic Radius - Anions
Nonmetals get larger when they become anions.
This is because they are gaining electrons, so their electron cloud gets bigger while the number of protons stays the same
With an additional electron, there is also additional electron repulsion
Ionic Radius - Cation
Metals get smaller when they become cations.
This because they are losing electrons, so their electron cloud get smaller
The nucleus exerts a stronger pull on fewer negative charges typically an energy level is lost.