Chapter 4: Thermal Physics
0.0(0)
0.0(0)
Card Sorting
1/16
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
1
New cards
**Heat**
thermal energy transmitted from one body to another
2
New cards
**Temperature**
a measure of an object’s internal thermal energy
3
New cards
three principal modes by which energy can be transferred
**Conduction**
**Convention**
**Radiation**
**Convention**
**Radiation**
4
New cards
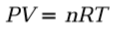
Ideal Gas Law
5
New cards
**Thermodynamics**
The study of the energy transfers involving work and heat, and the resulting changes in internal energy, temperature, volume, and pressure.
6
New cards
**Zeroth Law of Thermodynamics**
states that if Objects 1 and 2 are each in thermal equilibrium with Object 3, then Objects 1 and 2 are in thermal equilibrium with each other
7
New cards
**First Law of Thermodynamics**
states that energy (in the form of heat) is neither created nor destroyed in any thermodynamic system.
8
New cards
**Second Law of Thermodynamics**
defines the direction of time
9
New cards
**Heat Engines**
A device that uses heat to produce useful work
10
New cards
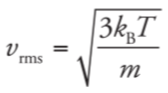
root-mean-square velocity formula
11
New cards
**an object can contain thermal energy**
an object doesn’t contain heat; heat is energy in transit
12
New cards
**Conduction**
Heat conducts from one point to another only if there is a temperature difference between the two objects.
13
New cards
**Convection**
As the air around a candle flame warms, it expands, becomes less dense than the surrounding cooler air, and thus rises due to buoyancy.
14
New cards
Absorption of the energy carried by these light waves defines heat transfer by ____________.
**radiation**
15
New cards
universal gas constant
**constant** ***R*** **= 8.31 J/mol·K**
16
New cards
**Boltzmann’s constant**
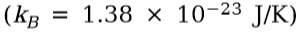
17
New cards
**Newton’s Second Law**
rate of change of momentum = force