Alkanes and Alkenes
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is the general formula for alkanes?
Alkanes have the general formula CnH2n+2
Why are alkanes are classified as saturated hydrocarbons?
Saturated: A molecule containing only single bonds between carbon atoms. For example, alkanes as described as saturated molecules.
How do you draw the structural and displayed formulae for alkanes with up to five carbon atoms in the molecule, and to name the unbranched-chain isomers?
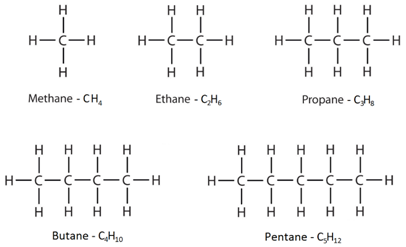
The displayed formulae show all the atoms and bonds drawn out.
The molecular formulae just show the number of each type of atom in the molecule.
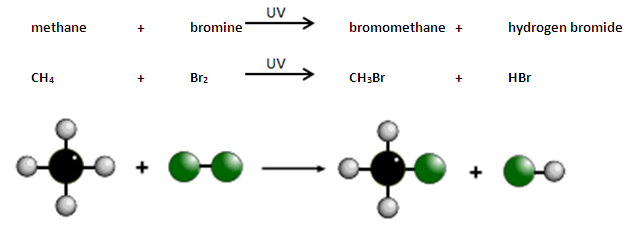
What is the reaction of alkanes with halogens in the presence of ultraviolet radiation?
Alkanes react with bromine in the presence of UV light, e.g. sunlight.
A hydrogen atom in the alkane is replaced by a bromine atom.
This is known as substitution.
What is the functional group of alkenes?
Alkenes are a homologous series of hydrocarbons which contain a carbon-carbon double bond. This double bond is shown in formulae as a double line.
The names of alkenes end with “ene”.
An example is ethene, the structural formula for which is CH₂ = CH₂
For a molecule with more than two carbon atoms, the position of the double bond within the molecule can vary as indicated by the name and the structural formula.
What is the general formula for alkenes?
Alkenes have the general formula CnH2n
Why are alkenes are classified as unsaturated hydrocarbons?
Unsaturated: A molecule containing a carbon-carbon double or triple bond. For example, alkenes as described as unsaturated molecules.
How do you draw the structural and displayed formulae for alkenes with up to four carbon atoms in the molecule?
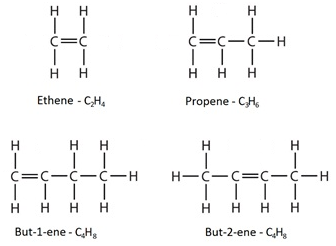
The displayed formulae show all the atoms and bonds drawn out.
The molecular formulae just show the number of each type of atom in the molecule.
What are the reactions of alkenes with bromine?
Alkenes react with bromine water. UV light is not required for this reaction.
The double bond is broken and the bromine atoms are added. This is an addition reaction.
During this reaction there is a colour change from orange to colourless.
For example:
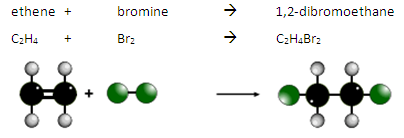
This is how we can test for the presence of an alkene or another type of unsaturated molecule.
How can bromine water be used to distinguish between an alkane and an alkene?
In the absence of UV light an alkane added to bromine water will not react: the bromine water will stay orange.
However, alkenes react with bromine water even without UV light. There will be a colour change of orange to colourless.