Biochemistry and Chemistry: Water, Reactions, Bonds, and Functional Groups
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
132 Terms
Water
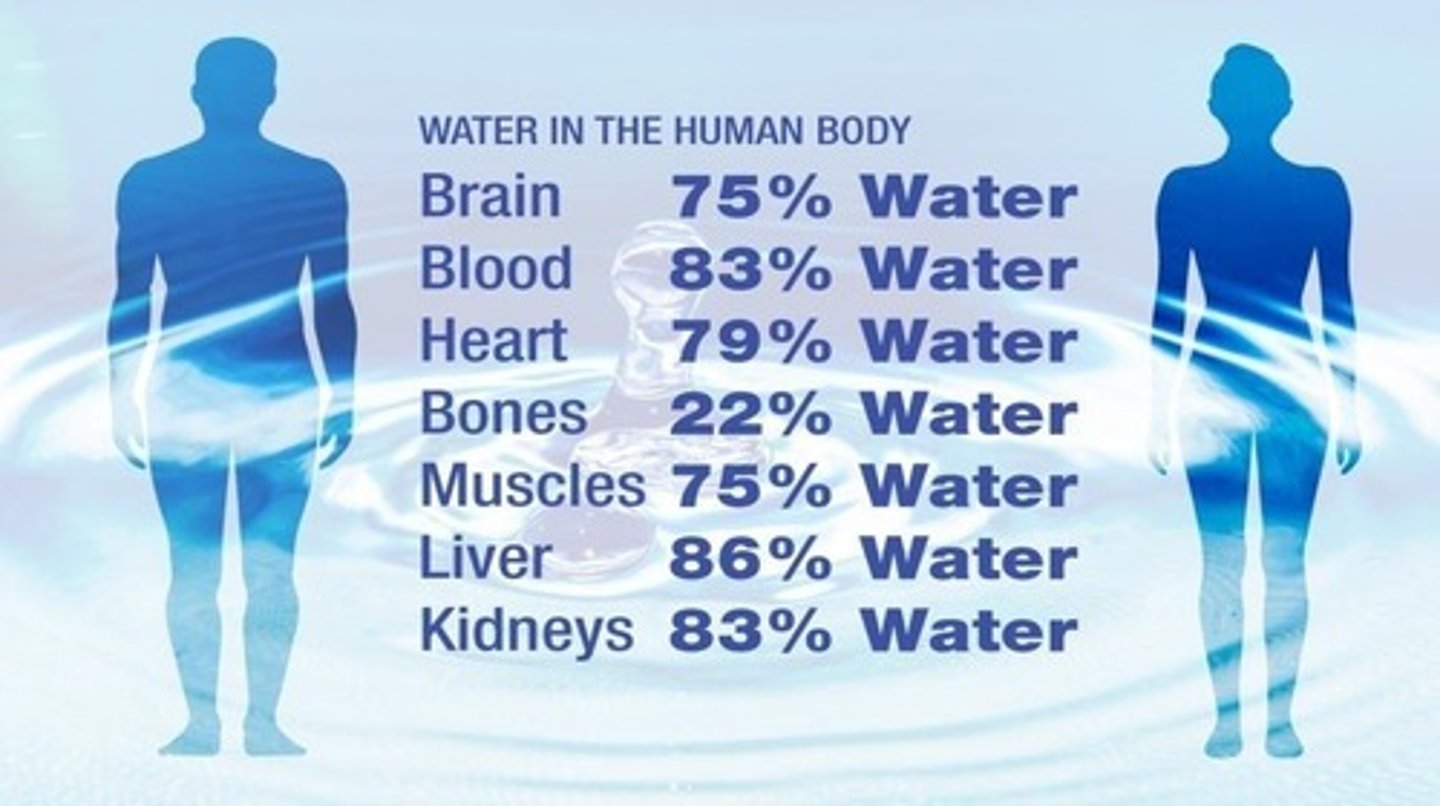
Water is the most abundant molecule in cells, accounting for 70% or more of total cell mass.
Polarity
The critical property of water is that it is a polar molecule.
Hydrophobic
Water fearing molecules (Nonpolar).
Hydrophilic
Water loving molecules (Polar).
Amphiphilic
Both water loving and fearing (Both polar/nonpolar).
Dehydration Synthesis (Condensation)
Joins monomers to form dimers/polymers.
Hydrolysis (Decomposition)
A reaction that breaks down compounds by the addition of water.
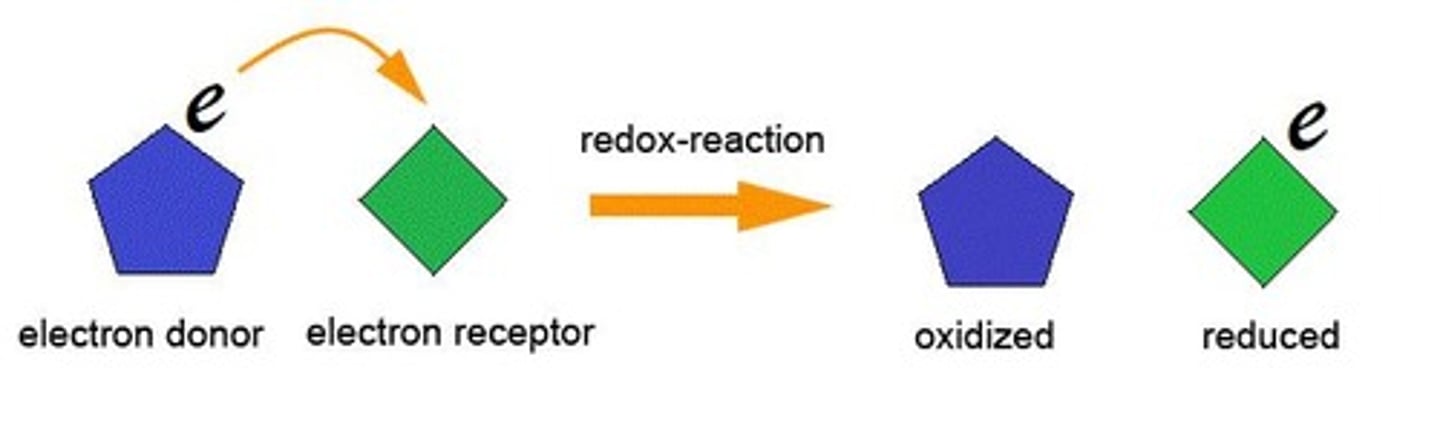
Redox
A type of reaction involving the transfer of electrons.
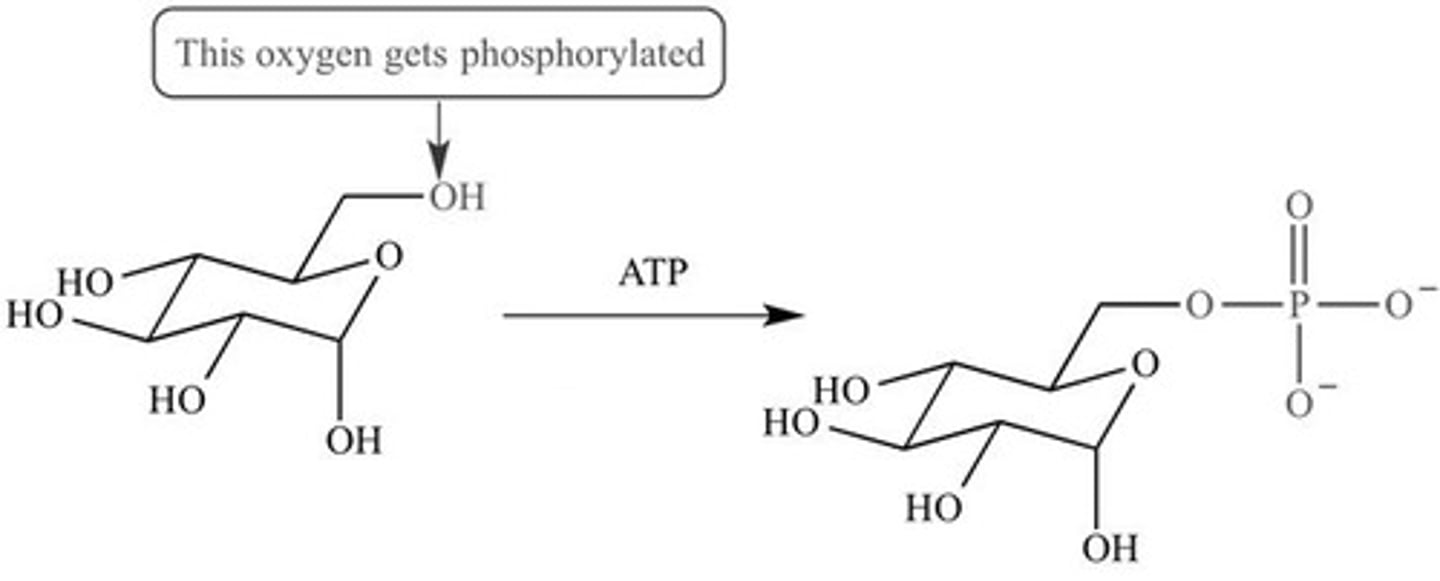
Phosphorylation
The addition of a phosphate group to a molecule.
Monomer
A single unit of a molecule.
Dimer
Two joined units of monomers.
Oligomer
A small molecule made of a few monomers (approximately 4-12).
Polymer
A large molecule made of many monomers.
Polymerization
The process of creating a polymer.
Joint Lubrication
Water lubricates and cushions joints.
Waste Removal
Water helps get rid of wastes through urination, perspiration, and bowel movements.
Cell Composition
Cells are composed of water, ions, and organic molecules.
Interactions of Water
Interactions between water and all other constituents of cells are of central importance.
Creation of Polymer
Creation of polymer is done by polymerization.
Water's Effect on Cells
Since most molecules are dissolved in water in the body, water has a profound effect on the cell.
Functional Groups
Certain functional groups may affect a molecule's polarity.
Bond Formation
Making and breaking bonds is important to forming molecules.
Dehydration Synthesis Reaction (DSR)
Joins monomers to form dimers/polymers.
Hydrolysis Reactions (HR)
Breaks a polymer into dimers/monomers.
Redox Reactions
Reduction-oxidation - Transfer of electrons between molecules.
Organic compounds
A large class of chemical compounds in which one or more atoms of carbon are covalently linked to atoms of other elements, most commonly hydrogen, oxygen, or nitrogen.
Hydrocarbon
An organic chemical compound composed exclusively of hydrogen and carbon atoms.
Hydrocarbons occurrence
Hydrocarbons occur naturally and form the basis of crude oil, natural gas, coal.
Hydrocarbon chain
A molecule that consists of hydrogen and carbon.
Structural formulas
A method of drawing hydrocarbons that shows the arrangement of atoms in the molecule.
Skeletal formulas
A method of drawing hydrocarbons where every 'kink' represents a carbon atom and hydrogen 'side groups' are not drawn.
Bonds in atoms
Every atom has the proper number of necessary bonds: Carbon: 4, Oxygen: 2, Hydrogen: 1.
Reactivity of functional groups
Functional groups are more reactive than hydrocarbons.
Hydrophilic functional groups
Functional groups that are polar and increase solubility in water.
Hydrophobic functional groups
Functional groups that are nonpolar and decrease solubility in water.
Functional groups in molecules
In a molecule, there may be multiple functional groups present.
Methyl group
A functional group represented as H3C- or -CH3 depending on its position in the molecule.
ATP
A molecule that contains numerous functional groups, important in energy transfer.
Electronegativity
A measure of the tendency of an atom to attract a bonding pair of electrons.
Polar molecule
A molecule that has a net dipole moment due to the presence of polar bonds.
Nonpolar molecule
A molecule that does not have a net dipole moment, typically due to symmetrical distribution of electron density.
Covalent bond
A chemical bond that involves the sharing of electron pairs between atoms.
Overall polarity of a molecule
Determined by the sum of the individual bond polarities and the molecular geometry.
Polarity symbols
Symbols used to indicate the direction of polarity in a molecule.
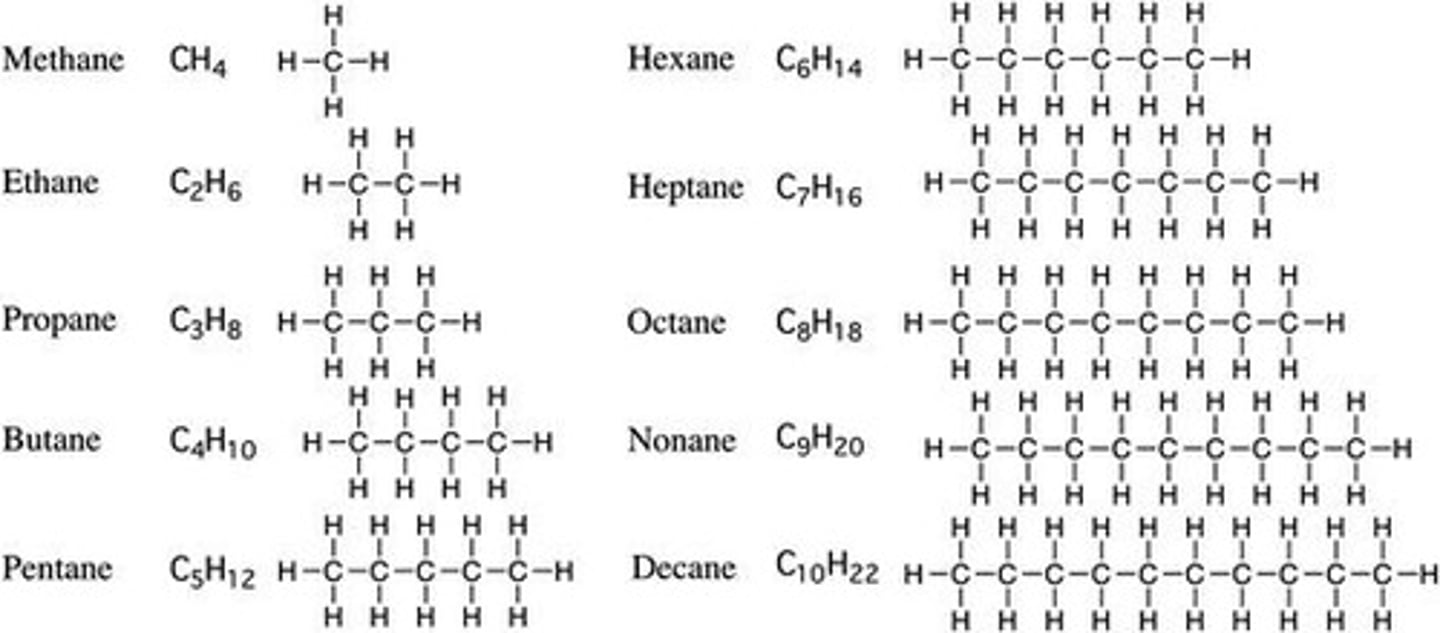
Hydrocarbons
M.E.P.B pentane hexane septane octane nonane decane
Electronegativity scale
A scale that ranks elements based on their ability to attract electrons. 0-0.5 non polar, 0.5-1.7 polar covalent, 1.7+ ionic
Chemical Bond
Force of attraction (FOA) between atoms, ions or molecules that enables the formation of chemical compounds.
Intramolecular Bonds
Bond(s) that holds atoms within a molecule together.
Intermolecular Bonds
Forces between molecules that allow them to (potentially) interact.
Intermolecular Forces
Three types collectively referred to as Van der Waals forces.
Dipole - Dipole Forces
FOA between the positive part of one polar molecule and the negative part of another polar molecule.
Dipole
A molecule with a partially positively charged end (δ+) and a partially negatively charged end (δ-).
Hydrogen Bonds
FOA between a H atom which is covalently bound to a more electronegative atom.
London Dispersion Forces (LDF)
Weak attractive force between any (and all) molecules caused by electrons randomly being on one side of an atom/molecule.
Dipole-Dipole Forces vs Hydrogen Bonds
Hydrogen bonds are usually stronger than dipole-dipole forces.
Temporary Dipole
A dipole that occurs due to the random distribution of electrons.
Ranking Intermolecular Force
LDF weakest, then it is dipole-dipole, then hydrogen bonds
Nonpolar Molecules and LDF
If a molecule is nonpolar, LDF are the only intermolecular forces.
Opposites Attract
In dipole-dipole interactions, δ+ is attracted to δ- between molecules.
Strong Intermolecular Force
Hydrogen bonds are a strong intermolecular force.
All Molecules Have LDF
All molecules have LDF, but if they can have DD or HB, those forces usually 'overpower' LDF.
Hydrophobic Forces
Another term for London Dispersion Forces.
Effect of Intermolecular Forces
Intermolecular forces only have an effect when molecules are close together.
London Dispersion
A type of intermolecular force that occurs between non-polar molecules.
Like dissolves like
Substances with similar chemical characteristics will dissolve in each other.
Polar solvents
Solvents that tend to dissolve polar solutes.
Non-polar solvents
Solvents that tend to dissolve non-polar solutes.
Polar substances
Substances that have a distribution of electrical charge leading to partial positive and negative charges.
Non-polar substances
Substances that do not have a significant charge distribution.
Intra- and intermolecular bonds
Intra- refers to bonds within a molecule, while inter refers to bonds between molecules.
Types of intermolecular bonds
Includes hydrogen bonds, dipole-dipole interactions, and London dispersion forces.
Strongest intermolecular bond
Hydrogen bonding is typically the strongest type of intermolecular bond.
Weakest intermolecular bond
London dispersion forces are typically the weakest type of intermolecular bond.
Always occurring intermolecular force
London dispersion forces occur regardless of the polarity of molecules.
Hydrogen bonding in water
Water molecules can do hydrogen bonding due to the presence of hydrogen atoms bonded to oxygen.
Hydrogen bonding in hexane
Hexane cannot do hydrogen bonding as it is a non-polar molecule.
Nonpolar Covalent Bonds
Equal sharing of valence electrons between two non-metal atoms.
Polar Covalent Bonds
Unequal sharing of valence electrons between two non-metal atoms.
Ionic Bonds
Bonds formed through the transfer of electrons from one atom to another.
Metallic Bonds
Bonds formed by the attraction between metal ions and delocalized electrons.
Solubility of polar molecules
Polar molecules tend to be soluble in polar solvents.
Solubility of non-polar molecules
Non-polar molecules tend to be soluble in non-polar solvents.
Intermolecular forces in water
Hydrogen bonds hold water molecules together.
Intermolecular forces in hexane
London dispersion forces hold hexane molecules together.
Drawing intermolecular forces
Intermolecular forces can be represented as dotted lines between molecules.
Polar Covalent Bonds (PC)
Unequal sharing of valence e-s between two non-metal atoms.
Differences in electronegativity (∆EN)
Can be used to predict bond type.
Small ∆EN
Expected when two atoms share electrons.
Large ∆EN
Expected when one atom steals an electron.
Electronegativity difference (∆EN)
Difference in electronegativities between two atoms.
NPC
Nonpolar covalent bond, indicated by a ∆EN of 0 - 0.5.
PC
Polar covalent bond, indicated by a ∆EN of 0.5 - 1.7.
I
Ionic bond, indicated by a ∆EN greater than 1.7.
KF
Example calculation of ∆EN resulting in 3.18.
O2
Example with ∆EN of 0, indicating a nonpolar covalent bond.
KCl
Example with ∆EN of 2.38, indicating an ionic bond.
CCl
Example with ∆EN of 0.6, indicating a polar covalent bond.
HCl
Example with ∆EN of 1.0, indicating a polar covalent bond.
OH
Example with ∆EN of 1.2, indicating a polar covalent bond.
Water (O-H Bond)
Example with ∆EN of 1.2, indicating a polar covalent bond.