Topic 1 - Quantization of Energy: Wave-Particle Duality and the Photo-Electric Effect
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Photons
Discrete packets (quantized) of light energy
Quantum Mechanics: Wave-Particle Duality
Discovered that:
Light (waves) shows particle-like properties.
Small particles (e.g., electrons) show wave-like (light) properties.
Photons — Photons absorbed/emitted by atoms, molecules, or surfaces when they increase/decrease in energy. This is the basis of spectroscopy.
When atoms/molecules gain energy → photon is absorbed
When atoms/molecules lose energy → photon is emitted
Electrons — Not point particles orbiting a nuclei but wavefunctions/orbitals with shape (s, p, d, f) and quantised energy (energy levels)
Orbitals/Wavefunctions
Specific regions around a nucleus where electrons are most likely to be found.
Photon Properties in Quantum Mechanics
Massless
Chargeless
Energy is quantized into discrete packets “quanta” proportional to frequency, as opposed to being continuous in classical mechanics
Photoelectric Effect - Hertz's Observation (1887)
Light of sufficiently high frequency ejects electrons from a metal surface, when shined upon it.
Key findings:
Below a frequency threshold → no electrons emitted
Threshold varies for different metals.
Increasing intensity (brightness) without meeting threshold → no effect
Contradicts classical physics as it proposed that electrons would be released when the intensity of light was further increased.
Intensity (Brightness)
Classical Mechanics: The amplitude of the wave.
Quantum Mechanics: The amount of photons hitting the surface per second.
Photoelectric Effect - Einstein’s Explanation
E = hν
Alternative formula: E = hc/λ
E in Joules (J)
v in Hertz (Hz)
h = 6.626 x 10^-34 J/s (Planck Constant)
Photon energy depends on (proportional to) frequency, not intensity.
Photon energy is inversely proportional to the wavelength.
Photoelectric Effect - Frequency Dependence Equation
E = hν
Alternative formula: E = hc/λ
E in Joules (J)
v in Hertz (Hz)
h = 6.626 x 10^-34 J/s (Planck Constant)
Wave Equation
c = λν (c = 2.998 × 10⁸ m/s)
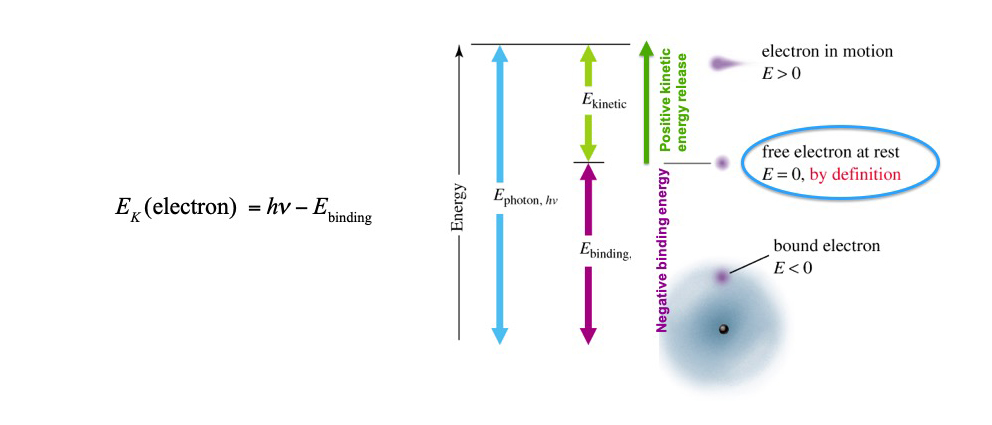
Photoelectric Effect - Electron Kinetic Energy Equation
Ek = Ephoton(hv) - Ebinding
Calculates the energy of the electron ejected from the atom (bound → free)
Photoelectric Effect - Electron Kinetic Energy's Atomic Structure
Binding energy (energy for an electron bound to a metal) depends on the atom/metal.
One excess photon energy beyond binding energy → is capable of liberating one electron = kinetic energy of ejected electron.
Insufficient energy = no electron ejected.
Just enough energy = electron ejected + small kinetic energy.
Plenty of energy = electron ejected + large kinetic energy.
Free electron at rest has zero kinetic energy, hence the binding energy has negative (-) value and the kinetic energy has (+) value.
Tips
The binding energy can also be called a work function.
Under certain conditions, the binding energy can also be referred to as ionization energy.