Required practical 2 - Measuring enthalpy changes
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
Outline the method for this practical:
This reaction in particular is exothermic.'
Anhydrous copper sulfate + aq → Copper sulfate solution
Weigh anhydrous copper sulfate amount 4 grams
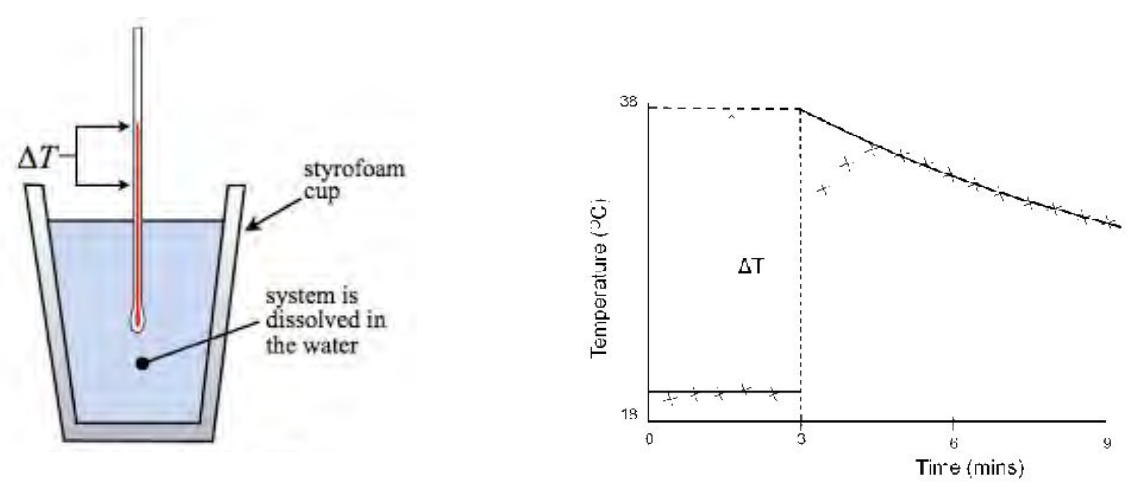
Place thermometer in polystyrene cup and record the temperature of 25cm cubed of deionised water for every 30 seconds
start the timer when adding the anhydrous copper sulfate (stir) and record temperature every 30 seconds for 4 minutes
Plot a graph with the temperature on y axis and time of x-axis. Draw two separate best fit lines, and one that joins the points after addition, extrapolate the lines to the fourth minute.
Use the graph to determine the temperature change at the fourth minute, which is the theoretical temperature rise as soon as addition of solid
Errors:
There is some heat transfer to or from the surrounding
The method assumes all solutions have the heat capacity of water
Some of the water could be evaporated
To improve accuracy:
Polystryene cup (if not stated in the question) should be used to reduce heat loss to the surrounding
A flame calorimeter could be used to improve accuracy
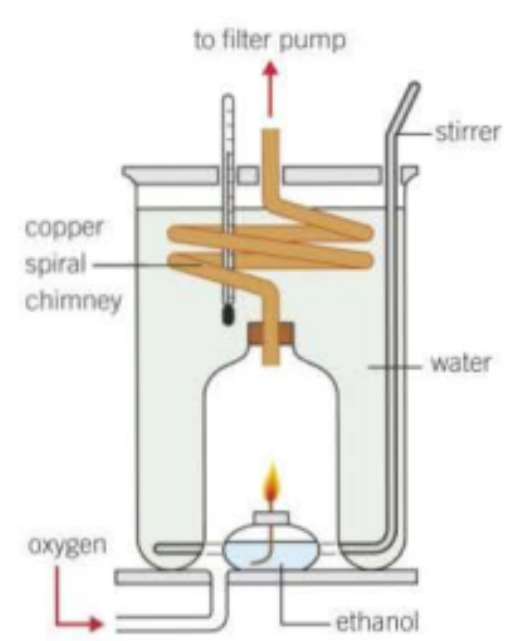
Spiral chimney of copper, good conductor of heat
Flame is enclosed
Fuel burns in pure oxygen instead of air
How does a flame calorimetry look like?