Chapter 16 Flashcards
1/9
Earn XP
Description and Tags
Flashcards on Common Ion Effect and Buffer Solutions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Common Ion Effect
The presence of a common ion suppresses the ionization of a weak acid or weak base.
Buffer Solution
A chemical system that resists pH changes by neutralizing added acid or base.
Henderson Hasselbalch Equation
The equation that helps to simplify solutions with the common ion effect.
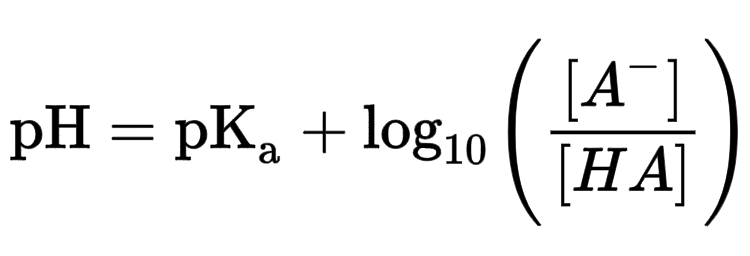
Buffer Capacity
How much acid or base a buffer can effectively neutralize.
Buffer Range
The pH range over which a particular acid and conjugate base can be effective as a buffer.
Weak Acid
Neutralizes added base in a buffer solution.
Conjugate Base
Neutralizes added acid in a buffer solution
Buffer Effectiveness
Most effective when the concentrations of acid and conjugate base are equal and high.
pKa
The effective range for a buffering system is generally one pH unit on either side of this value.
Common Ion Effect
Follow Le Chatelier’s Principle