CHEM231 Chapters 1 + 2
1/54
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
Electronegativity Trend
Increases from left to right, bottom to top
S Orbital
Circle

P Orbital
Rounded figure eight
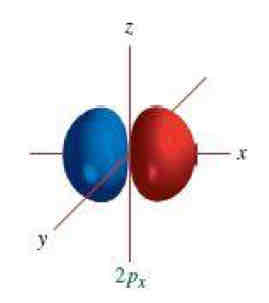
Dipole Moment
Measurement of dipole
How does electronegativity effect dipole moment?
Increased electronegativity leads to increased dipole moment
How does number of bonds effect dipole moment?
Increasing number of bonds increases dipole moment
What are the valence electrons of N, O, F?
5, 6, 7
How to get formal charge?
Valence electrons - electrons owned by atom
Line Drawing
Ends of lines and bends are carbons, hydrogen is inferred, only heteroatoms and their Hs are drawn
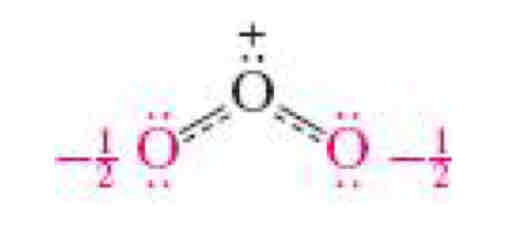
What is resonance?
Electron delocalization
Resonance
Moving around uneven electrons / lone pairs
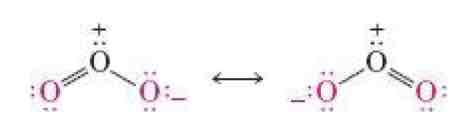
Resonance hybrid
Even sharing with the use of a partial bond
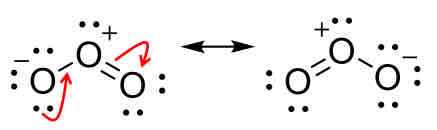
Curved arrows
Shows the movement of electrons
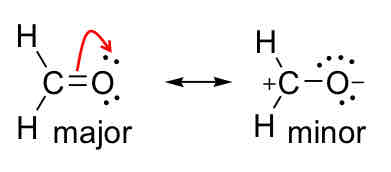
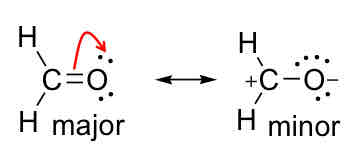
Major / Minor Contributors
Major is usually more stable and more commonly found, minor is more unlikely
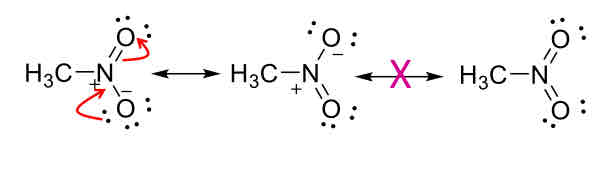
Resonance rules
Connectivity stays the same
Number of electrons and net charge is the same
Number of unpaired electrons is the same
Any structure exceeding the octet rule for 2nd row elements is not valid
How to predict the major contributor?
Structure with more covalent bonds contribute more
With octet satisfied, major structure has least amount of charge separation
With octet satisfied, major contributor has negative charge on most electronegative element
Increasing force of repulsion between electron pairs
Bonded pairs (least) → unshared pair to bonded pair → unshared pairs (most)
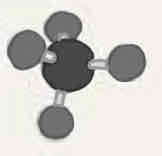
Molecular geometry: Methane (CH4)
109.5°, four bonded pairs, tetrahedral, tetrahedral
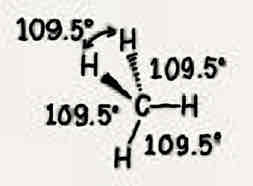
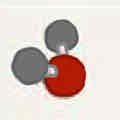
Molecular geometry: Water (H2O)
105°, two bonded pairs + two unshared pairs, tetrahedral, bent
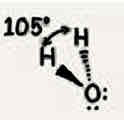
Molecular geometry: Ammonia (NH3)
107°, three bonded pairs + one unshared pair, tetrahedral, trigonal pyramid
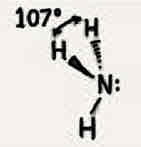
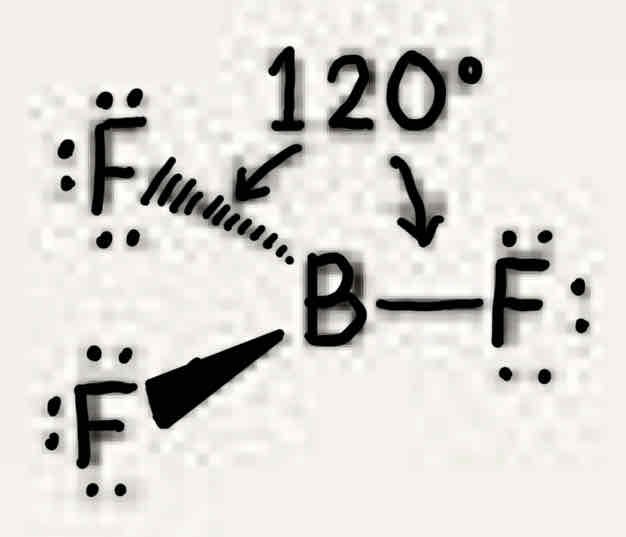
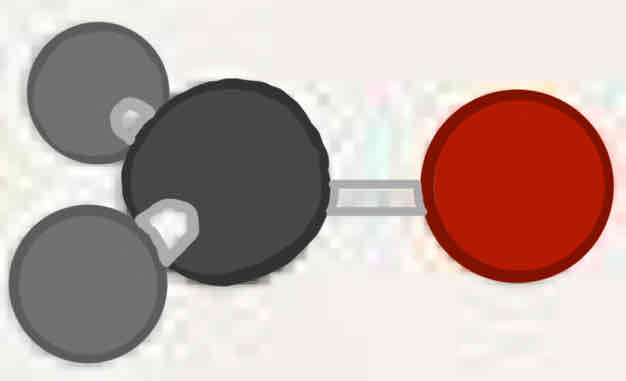
Molecular geometry: Boron trifluoride (BF3)
120°, three bonded pairs, trigonal planar, trigonal planar
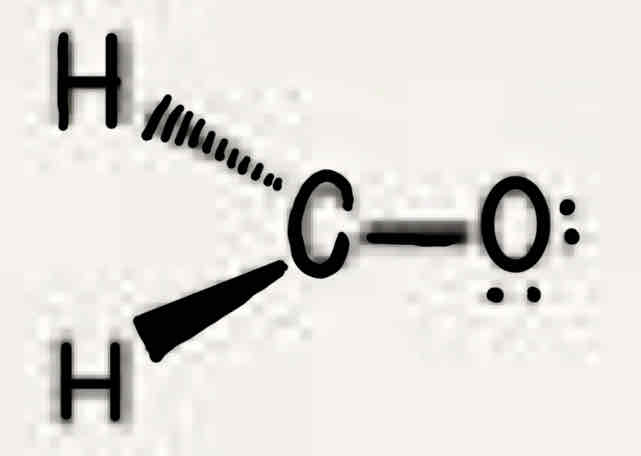
Molecular geometry: Formaldehyde (H2CO)
120°, two bonded pairs + one double bond (one bonded pair), trigonal planar, trigonal planar
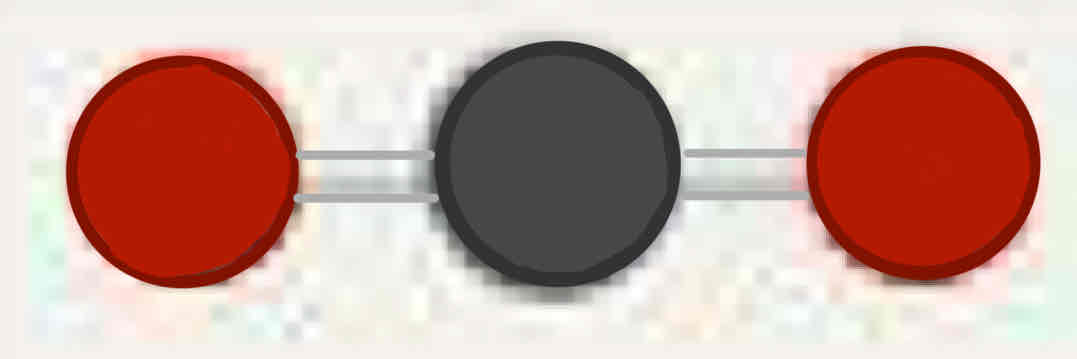
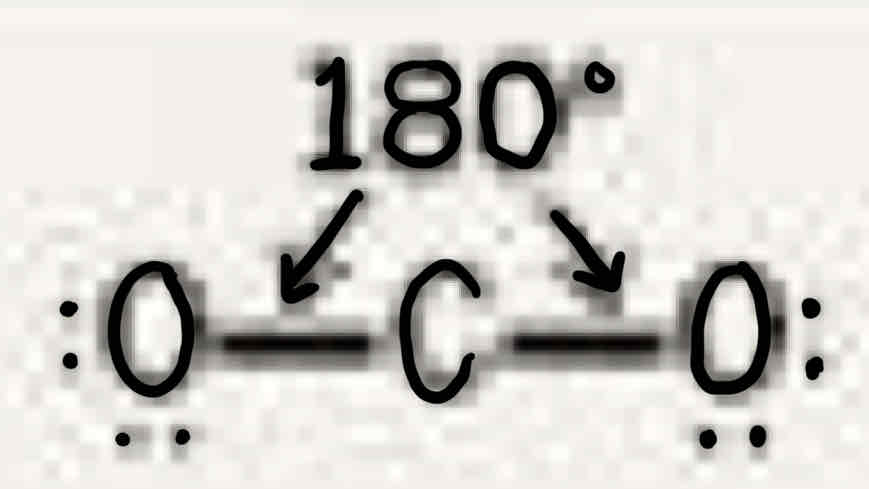
Molecular geometry: Carbon Dioxide (CO2)
180°, two double bonds (two bonded pairs), linear, linear
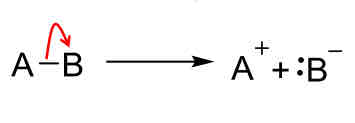
Reaction mechanisms: Dissociation
Bond broken
Reaction mechanisms: Formation
Bonds form
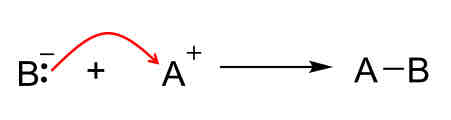
Reaction mechanisms: Substitution
Replacing something in a bond
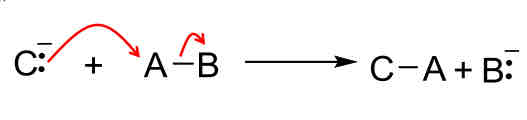
Reaction mechanisms: Acid-Base Reactions
Acid or base being added in
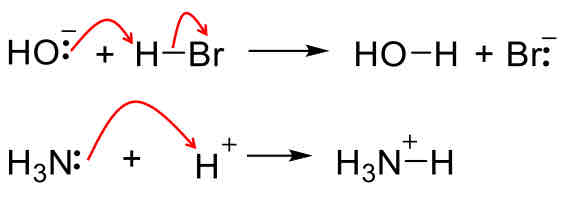
Brønsted Acid
Proton donor
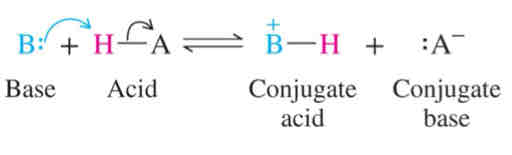
Brønsted Base
Proton acceptor
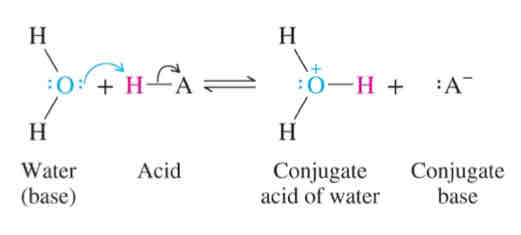
pKa =
-log (Ka)
Acidity strength: pKa
The more negative pKa → stronger the acid
Acidity strength: Periodic Table
More acidic down the column (F<Cl<Br<I), row follows electronegativity trend
Inductive effects
Inductive withdrawing groups near the conjugate base anion increases the acidity
Acid-Base Equilibria
Reaction forms weaker acid/base, equilibrium shifts to weaker acid
Lewis Acid
Electron acceptor
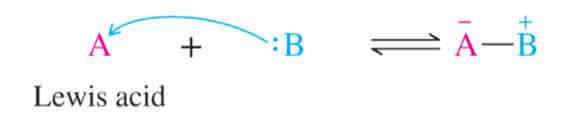
Lewis Base
Electron donor

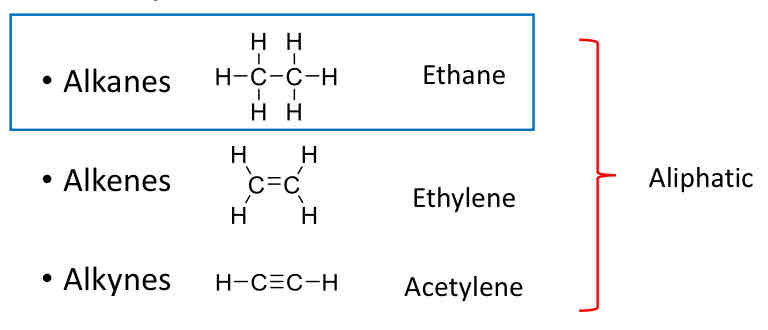
Classes of Hydrocarbons: Aliphatic
Alkanes (Ethane), Alkenes (Ethylene), Alkynes (Acetylene)
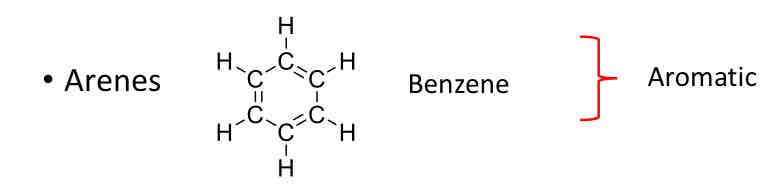
Classes of Hydrocarbons: Aromatic
Arenes (Benzene)
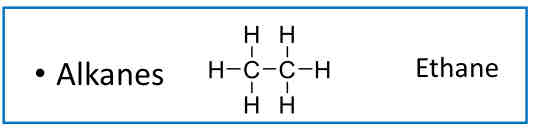
“Saturated” Hydrocarbons
Alkanes (Ethane)
H2 (Nuclear Distance)
Ideal inter nuclear distance → 74 pm, potential energy → -435 kJ/mol (-104 kcal/mol)
kJ/mol or kcal/mol?
Interchangeable
Linear alkane formula
C(n)H(2n+2)
sp3 Hybridization
Sigma bond (single line), least energy (25:75)
sp2 Hybridization
1 sigma bond + 1 pi bond (double bond), mid energy (33:66)
sp Hybridization
1 sigma + 2 pi bonds, highest energy (50:50)
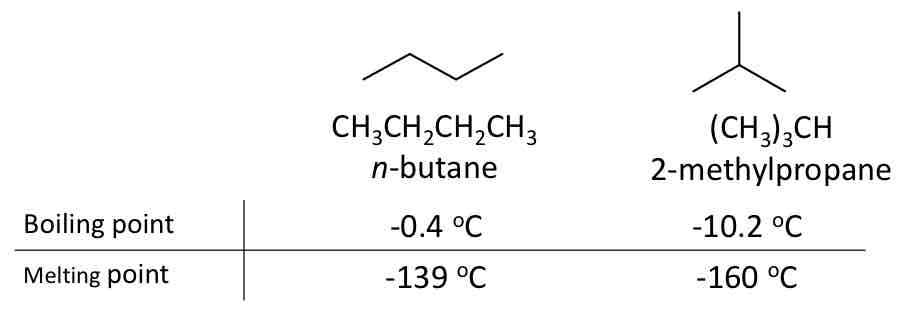
Butane Isomers (C4H10)
Constitutional isomers
Same molecular formula
Different connectivity
Different physical properties
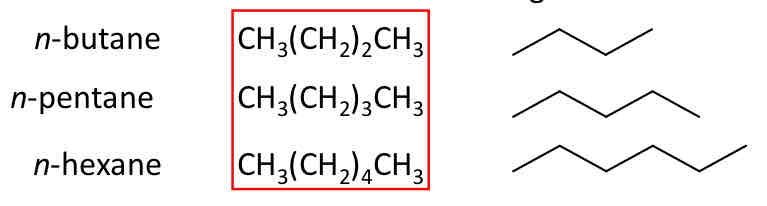
n-Alkanes
Condensed structural formula of higher n-alkanes
Pentane Isomers (C5H12)
Constitutional isomers: n-Pentane, isopentane, neopentane
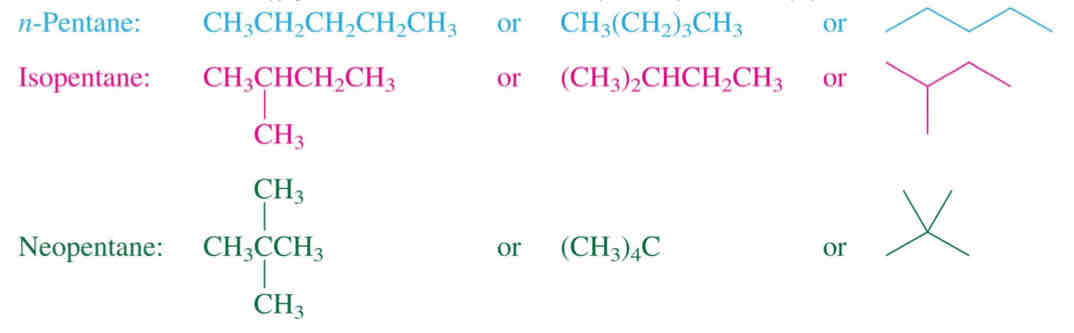
Physical properties of alkanes
Low melting points
Poor solubility in water
Boiling points lower than analogous amines or alcohols
Boiling points change among isomers
Acidity of hydrocarbon C-H bonds
Alkanes are weakest acids known
Combustion of alkanes
Combustion relates to stability of isomers, higher kJ/kcal the more unstable
Oxidation
Increase in bonds to electronegativity elements
Reduction
Increase in bonds to less electronegative elements
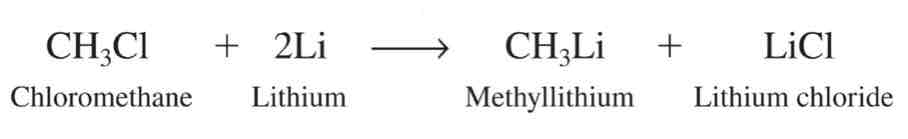
IUPAC Names of Unbranched Alkanes
Methane
Ethane
Propane
Butane
Pentane
Hexane
Heptane
Octane
Nonane
Decane
Undecane
Dodecane
IUPAC Naming
Count parent chain (longest chain) (add cyclo- if it’s a shape)
Determine substituants (1-methyl, 2-ethyl, 3-propyl, 4-isopropyl)
Name alphabetically (#-wordchain)
Add di, tri, or tetra if multiple of one substituants (#,#-diwordchain)