Grignard's Reagent
1/47
Earn XP
Description and Tags
Preparation and Properties
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
Grignard’s Reagent chemical formula?
R- Mg+X
Alkyl Magnesium Halide
Preparation of Grignard’s Reagent?
R-X + Mg —(in presence of dry ether)→ R=MgX
When preparing Grignards’ Reagent what are the reactants?
R-X and Mg
Alkyl Halide and Magnesium
What solvent should the preparation of Grignard’s Reagent take place in
Dry Ether
What is the mechanism of the reaction of preparation of Grignard’s Reagent?
Magnesium has 2 electrons in the outermost shell.
in R-X, R has partial positive charge and X has partial negative charge. Magnesium gives its two electrons to the electrophile, which is R. In this process, the electrons from the covalent bond R-X go to X, giving X a negative charge.
R-Mg becomes a covalent bond, and Mg-X becomes an ionic bond.
In R-MgX, what is the nature of the bond between R and Mg?
It is a covalent bond.
In R-MgX, what is the nature of the bond between Mg and X?
It is an ionic bond.
RMgX is the source of a _________ (nucleophile/electrophile) or ________ (acid/base).
It provides a ________ (carbanion/carbocation).
RMgX is the source of a nucleophile (R-) or base.
R provides a carbanion.
RMgX acts as ______ (acid/base).
RMgX acts as base.
RMgX acts as _______ (nucleophile/electrophile).
RMgX acts as nucleophile.
R- _______ H+. (accepts/donates)
R- accepts H+.
R- _______ acidic hydrogen. (accepts/donates)
R- accepts acidic hydrogen.
CH3-CH2-MgCl on reaction with water gives?
CH3-CH3 + Mg(OH)Cl
CH3-CH2-MgCl on reaction with R-OH gives?
CH3-CH3
CH3-CH2-MgCl on reaction with ether gives?
It doesn’t give anything. No reaction.
CH3-CH2-MgCl on reaction with CH3-O-CH3 gives?
It doesn’t give anything. No reaction.
CH3-CH2-MgCl on reaction with H2O gives?
CH3-CH3 + Mg(OH)Cl
CH3-CH2-MgCl on reaction with alcohol gives?
CH3-CH3
CH3-CH2-MgCl on reaction with acetic acid gives?
CH3-CH3
CH3-CH2-MgCl on reaction with CH3COOH gives?
CH3-CH3
CH3-CH2-MgCl on reaction with NH3 gives?
CH3-CH3
CH3-CH2-MgCl on reaction with (CH3)3N gives?
It doesn’t give anything. No reaction.
CH3-CH2-MgCl on reaction with R-SO3H gives?
CH3-CH3
CH3-CH2-MgCl on reaction with phenol gives?
CH3-CH3
What role does ether play in the preparation of Gridnard’s reagent?
It is a solvent, used to dissolve the reactants by coordination.
What is the order of easiest formation of Grignard’s Reagent?
RI _ RBr _ RCl
RI > RBr > BCl
When CH3MgBr reacts with CH3COCl what do we get?
Explain the mechanism as well.
CH3COCH3 + MgBrCl
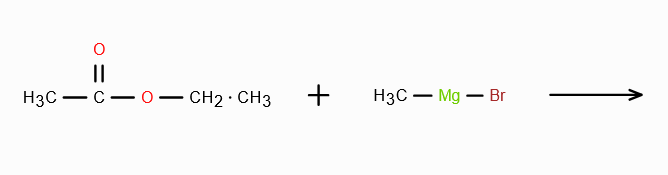
Explain the mechanism as well.
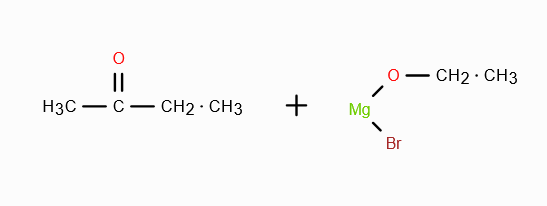
Explain the mechanism where Grignard’s Reagent acts as a nucleophile.
R- is a nucleophile and attacks a positive centre, usually the C doubly bonded with O in a ketone group. The C either breaks one bond with the O, making the O negatively charged so Mg+X attaches itself to it, or C breaks a bond with a leaving group which attaches with Mg+.
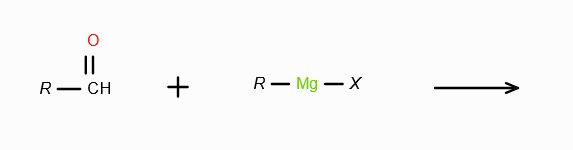
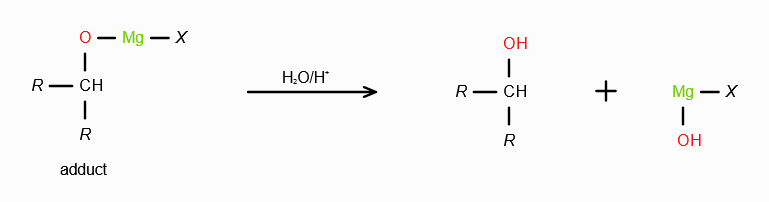
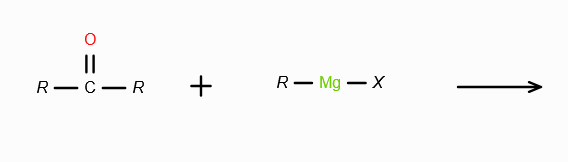
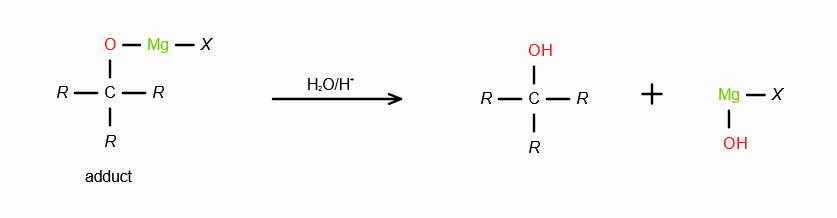
What does RMgX need to react with to make a 2o alcohol?
An aldehyde. On reaction with RMgX, an aldehyde forms an adduct, which gets reduced to a second degree alcohol in acidic medium.
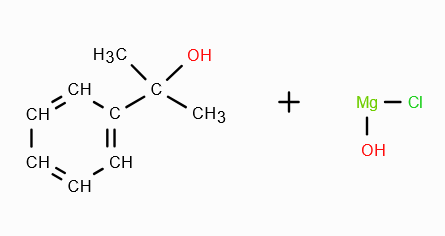
What does RMgX need to react with to make a 3o alcohol?
A ketone. On reaction with RMgX, a ketone forms an adduct, which gets reduced to a third degree alcohol in acidic medium.
When Grignard’s Reagent reacts with water, does it act as a base or a nucleophile?
It acts as a base.
When Grignard’s Reagent reacts with CH3COCl, does it act as a base or a nucleophile?
It acts as a nucleophile.
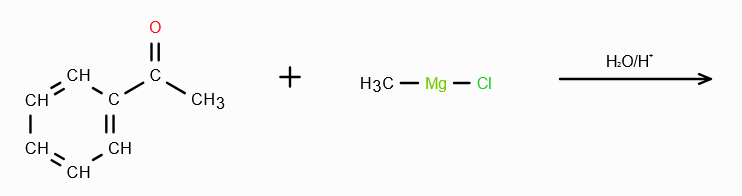
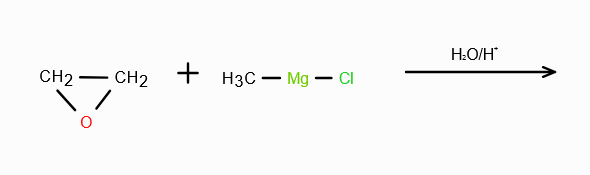
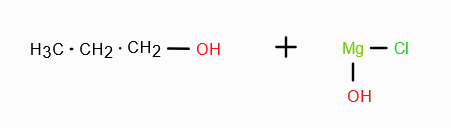
RMgX + H2CO/H3O+ →
RCH2—OH
RMgX + (i)O2 (ii) H3O+ →
R-OH
RMgX + HCOOEt/H3O+ →
R2CHOH
RMgX + RCOR/H2O →
R3C-OH
RMgX + RCN/H2O →
RCOR
RMgX + X2 →
R-X
RMgX + ClCOOEt →
RCOOEt
RMgX + Cl-NH2 →
R-NH2
What do you need to react with the Grignard’s Reagent if you want to make a primary amine?
X-NH2
RMgX + R-X →
R-R
What is the significance of having water as the medium during reactions with Grignard’s Reagent?
To satisfy the valency of the leaving group by adding a proton, and satisfy the valency of MgX by adding -OH.