Pathological basis of disease exam 1 -- Cell injury
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Cell membranes are damaged by 4 things:
ROS/free radicals
Decreased phospholipid synthesis (ATP, hypoxia)
Phospholipid breakdown
Cytoskeletal abnormalities
Decreased ATP —> _______ —> ________ (for membrane-bound organelles)
Ribosomes detach, decreased protein synthesis
Mitochondrial damage results in:
Leakage of pro-apoptotic proteins and decreased energy production. Loss of membrane potential. Can generate both necrosis and apoptosis
Cells respond to stress/injury in 3 ways:
Adapt
Degenerate
Die
Necrosis is always _____, apoptosis can be _____
Pathogenic, programmed
4 targets of cell injury
Integrity of cell membranes
Aerobic respirations via mitochondrial oxidative phosphorylation for ATP production
Protein synthesis
Integrity of nucleus
Hypoxia
Oxygen is insufficient at the tissue level to maintain adequate homeostasis
5 cell types that are sensitive to hypoxia and cell swelling
Neurons
Cardiac myocytes
Renal proximal tubular epithelium
Hepatocytes (liver)
Endothelium
Why is the renal proximal tubular epithelium sensitive to hypoxia and swelling
High concentration of sodium potassium ATPases pumped against the gradient so it needs a lot of oxygen and ATP
Why is endothelium sensitive to hypoxia and swelling
It does not recieve oxygen from the blood that passes by (gets it from vasculature instead), very sensitive to low oxygen
Reversible cell injuries
Mild swelling of cells and organelles
Membrane alterations (blebbing, loss of microvilli)
Ribosomes detach from rough ER
Chromatin clumping
Fatty change
Mitochondrial changes
Nuclear alterations
CELL MEMBRANE STILL INTACT
Irreversible cell injuries
Marked swelling
Lysosome disruption
Amorphous deposit in mitochondria
Membrane disruption (causes necrosis)
Nuclearr changes (pyknosis)
More myelin figures
4 characteristics of acute cell swelling
Mild damage to cell membranes
Hypoxia
Decreased energy production
Injury to enzymes regulating membrane ion channels
3 causes of ATP depletion
Reduced supply of oxygen and nutrients
Mitochondrial damage
Some toxins
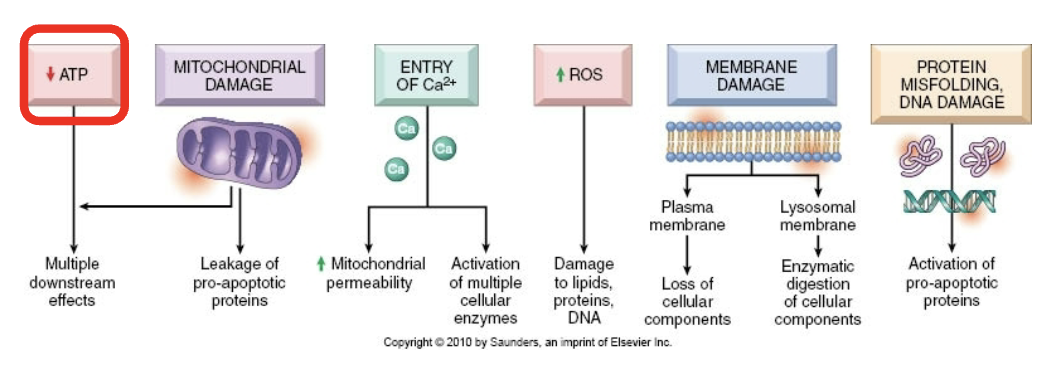
Decreased ATP leads to:
Failure of Na/K and Ca pump
Detachment of ribosomes, fewer proteins
Failure of glycolysis, lowe pH
Accumulation of misfolded proteins
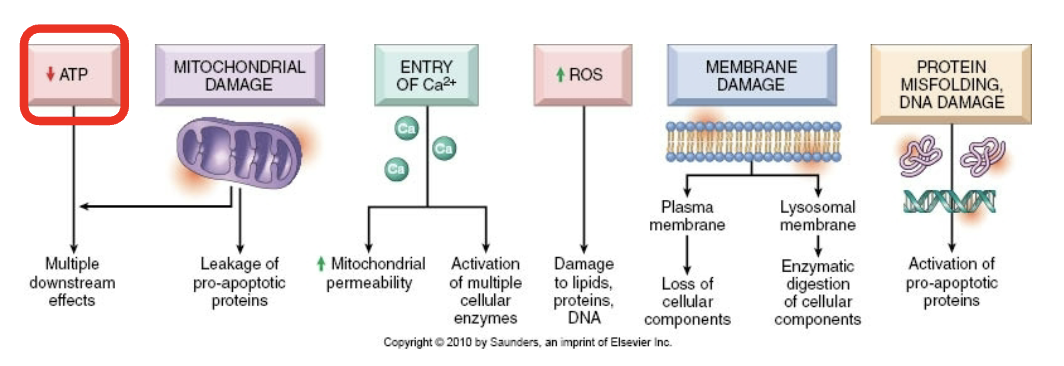
4 causes of mitochondrial damage
Increased intracellular Ca+
Free radicals/ROS
Hypoxia
Toxins
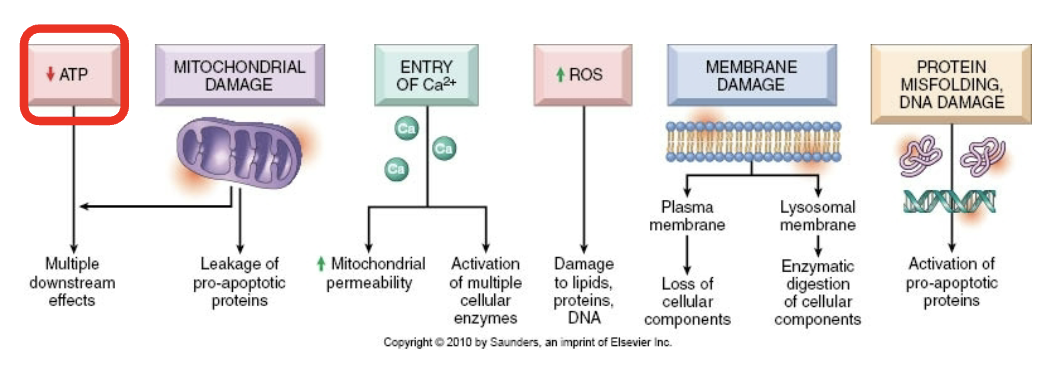
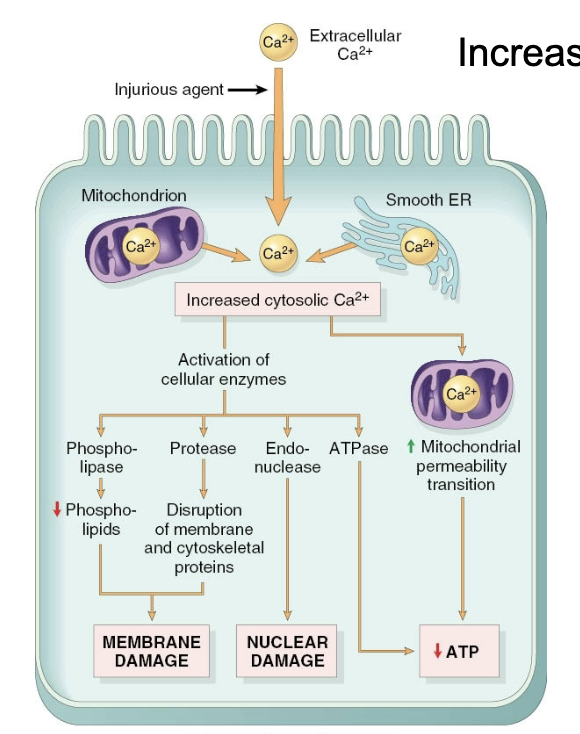
Consequences of calcium increase
Activation of phospholipases, proteases, endonucleases, ATPase
Leads to necrotic and apoptotic pathways
Origins of ROS
Inflammation
Decreased removal/scavenging
Radiation
Drugs
Generated in normal cellular processes and in response to ^ stressors
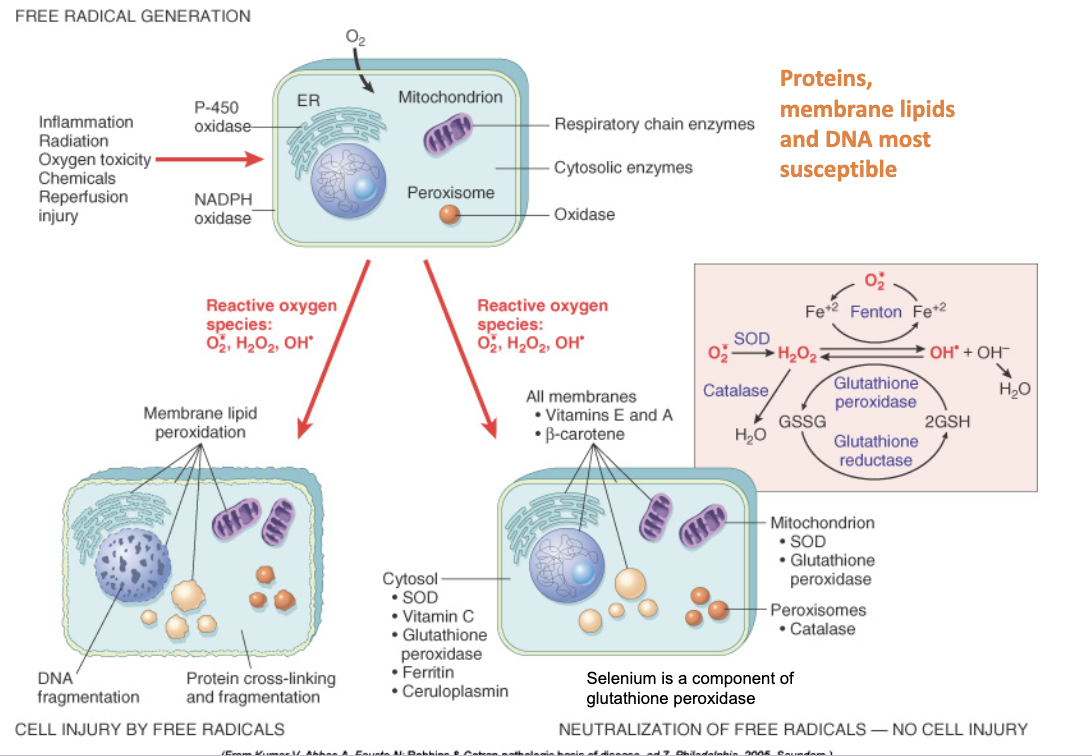
Antioxidant mechanisms in the mitochondria
Removes free radicals
Effects of ROS in cell injury/death
Disruption of plasma membrane, organelles
Loss of enzymatic activity, abnormal folding
Mutations, breaks
White muscle disease is caused by:
ROS (Lack of vitamin E and selenium —> can’t perform reducatase cycle)
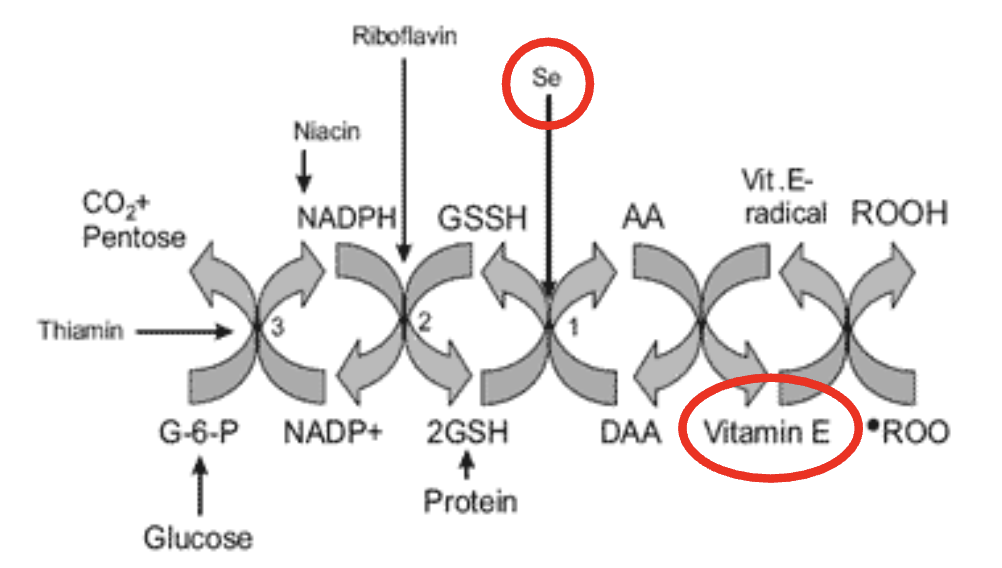
4 causes of membrane damage
Free radicals
ATP depletion
Bacterial toxins
Viruses
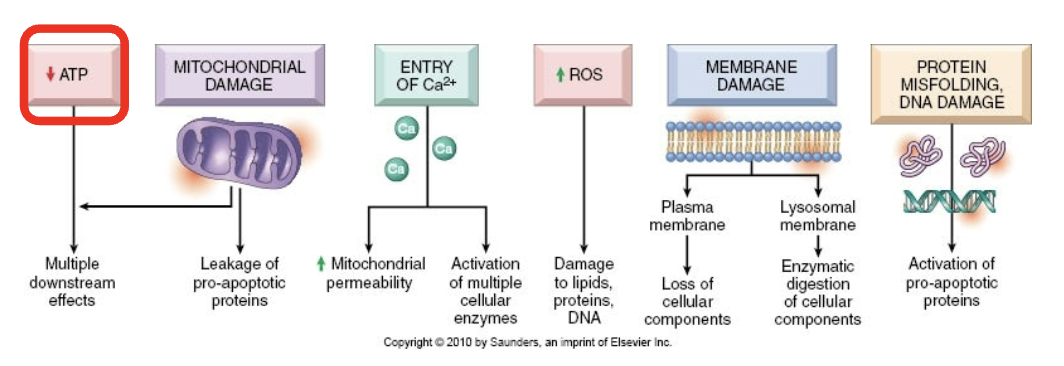
Consequences of membrane damage in mitochondria
Mitochondrial membrane becomes more permeable —> decreased ATP and apoptotic triggering proteins
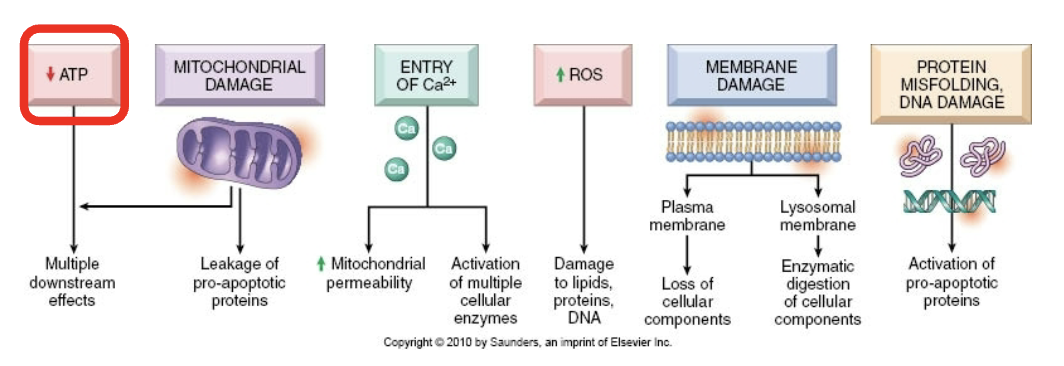
Consequences of membrane damage in plasma membrane
Influx of Na+, water, and Ca++, loss of K+ and other cellular subsstrates
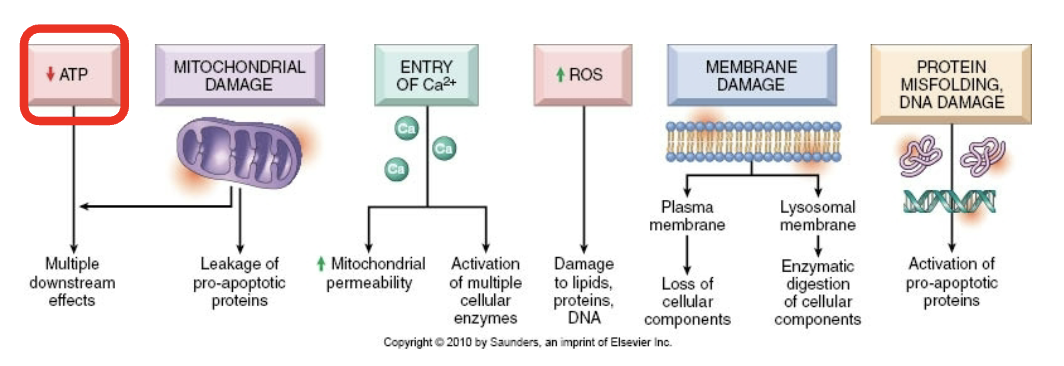
Consequences of membrane damage in Lysosomal membrane
Leakage of enzymes that digest protein, DNA, RNA, and glycogen
When does reversible damage become irreversible?
When there is lysosome disruption, mitochondrial dysfunction, and severe membrane imbalance
Necrosis
Inflammatory cell death
Karyorhexis
Type of nuclear change where pyknotic nucleus undergoes fragmentation
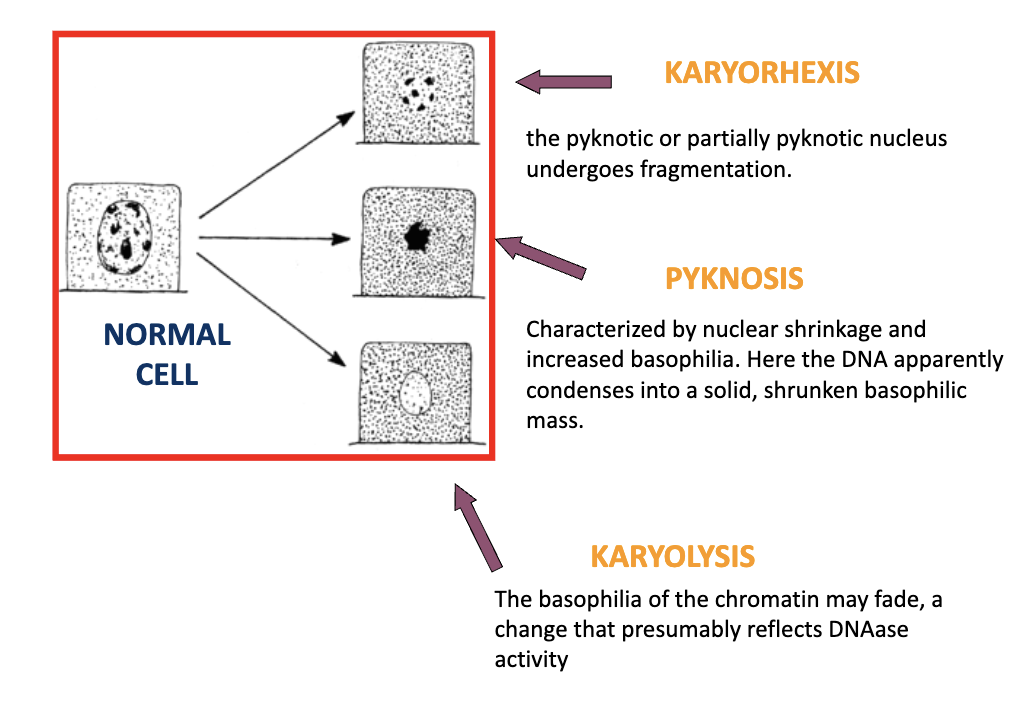
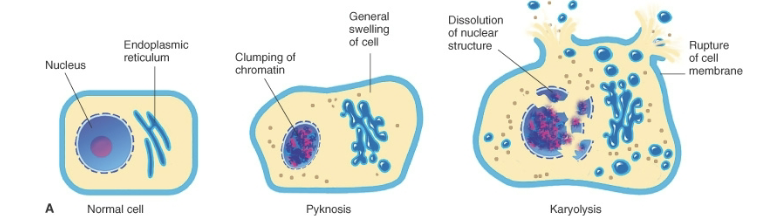
Pyknosis
Type of nuclear change characterized by nuclear shrinkage and increased basophilla. DNA condenses into a shrunken, solid basophilic mass
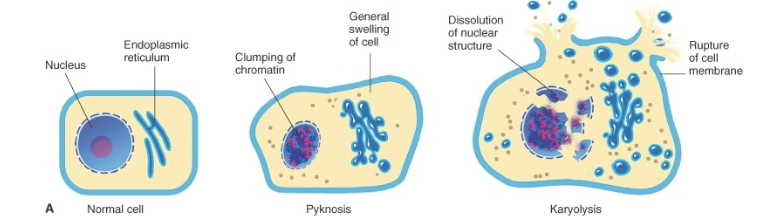
Karyolysis
Basophilia of the chromatin may fade, a change that presumably reflects DNAase activity
Coagulative necrosis
Type of necrosis
Architecture is preserved
Enzyme damage
Caused by ischemia, bacterial exotoxins, chemical toxins
Classic necrosis of the kidney
Lack of blood supply causing lesions = infarction
Liquefaction necrosis
Type of necrosis
Digestion of dead cells
Bacterial infections and WBCs
Central nervous system ischemia
Often happens in the brain
Caseous necrosis
Type of necrosis
Loss of architecture
Tuberculosis/myobacteria/corynebacteria
Birds and reptiles develope this die to low levels of myeloperoxidase in their heterophils. Can’t digest many bacteria
Fat necrosis
Type of necrosis
Release of activated pancreatic lipase into fat
Diets high in fatty acids and low in antioxidants
Chalky-white
Classically pancreatitis
Gangrene
Condition resulting from the decay of body tissue, often due to a lack of blood flow or bacterial infection. Initial lesion is coagulation necrosis
Dry gangrene
Coagulation necrosis due to infarction followed by mummification. Tissue dries out and bacteria can’t grow. Includes frostbite
Infarction
Obstruction of the blood supply to an organ or region of tissue leading to tissue death
Moist/wet gangrene
Necrotic tissue with further degradation by bacteria, causing it to rot
Gas gangrene
Similar to moist gangrene, populated by anaerobic bacteria like clostridium. Penetrating wounds and necrotic tissue are good environments for anaerobes
Capsases
Enzymes that degrade cellular components. Important in apoptosis
Physiological apoptosis
Type of apoptosis characterized by
Embryogenesis (“programmed cell death”
Hormonal
Self-reactive lymphoctes
Inflammatory cells
Pathological apoptosis
Type of apoptosis characterized by
DNA damage
Accumulation of misfolded proteins
Virus infections (such as adenovirus and HIV)
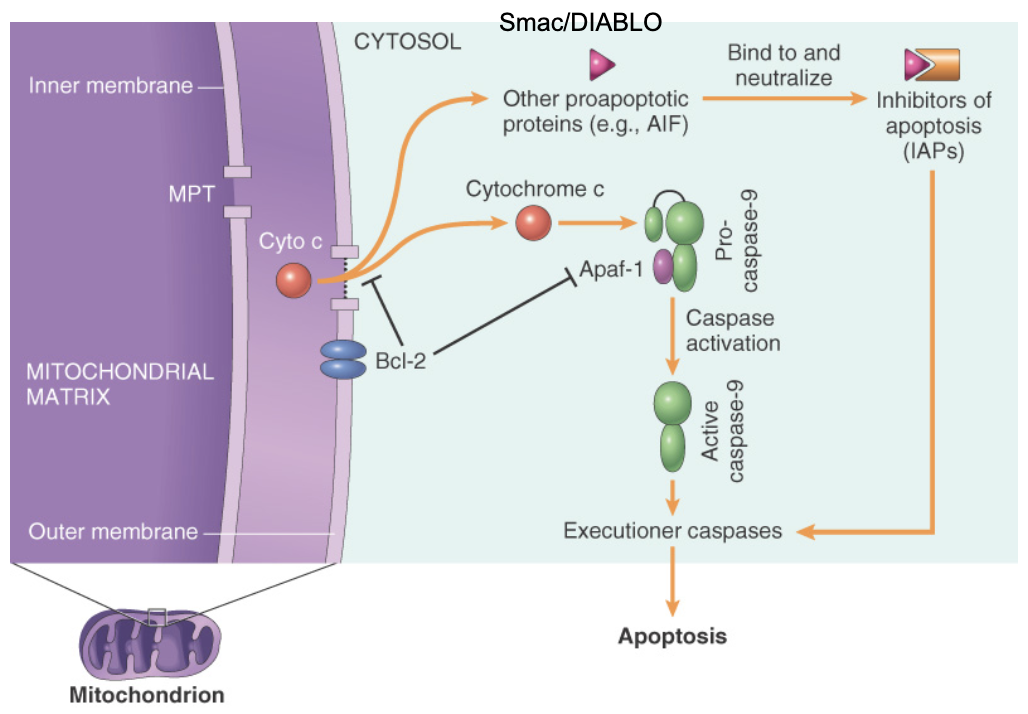
Intrinsic pathway to apoptosis
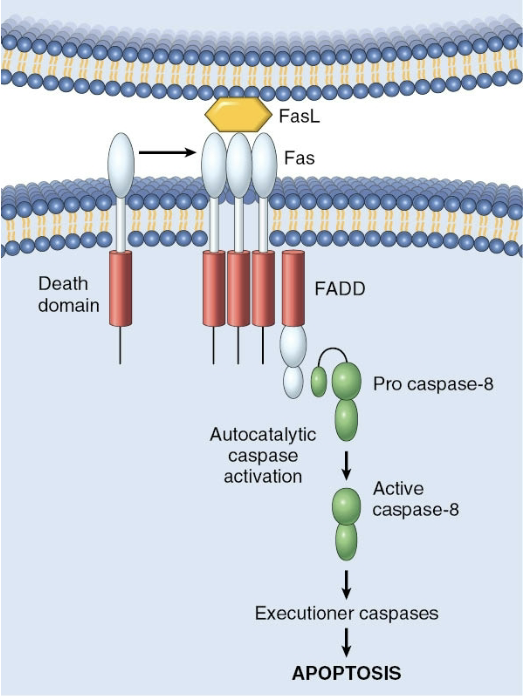
Extrinsic pathway to apoptosis
Apaf-1
Pro-apoptotic factor in intrinsic pathway. Activates caspase 9
FLIP
Anti-apoptotic factor in extrinsic pathway. Binds to and neutralizes procaspase 8
Bcl-2 and Bcl-x
Anti-apoptotic factors in intrinsic pathway. Control membrane permeability (more permeability = release of pro-apoptotic factors)
Smac/DIABLO
Pro-apoptotic factor. Binds to + neutralizes IAPs in intrinsic pathway
Cytochrome C
Pro-apoptotic factor, binds to and activates caspase 9 in intrinsic pathway
Bax and Bak
Pro apoptotic factor, creates channel in mitochondria, binds/blocks function in Bcl-2 family in intrinsic pathway
IAPs (inhibitors of apoptosis)
Anti-apoptotic factors. Block activation of caspases in intrinsic pathway
Bid, Bim, Bad
Pro-apoptotic factor, activates Bax and Bak
Mcl-1
Anti-apoptotic factor in intrinsic pathway