Famous Scientist Atomic Theory
0.0(0)
Card Sorting
1/9
Earn XP
Description and Tags
Last updated 1:12 AM on 9/6/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
John Dalton
5 Parts of Dalton's Theory
Model is known as the **Billiard Ball.**
1. All matter is made up of tiny, indivisible particles called atoms. **(FALSE)**
2. Atoms of the same element are identical. **(FALSE - ISOTOPES)**
3. In a chemical reaction, atoms are combined, rearranged, or separated.
4. Atoms of different elements chemically combine to form a whole number ratio called compounds
5. Atoms cannot be created or destroyed
Model is known as the **Billiard Ball.**
1. All matter is made up of tiny, indivisible particles called atoms. **(FALSE)**
2. Atoms of the same element are identical. **(FALSE - ISOTOPES)**
3. In a chemical reaction, atoms are combined, rearranged, or separated.
4. Atoms of different elements chemically combine to form a whole number ratio called compounds
5. Atoms cannot be created or destroyed

2
New cards
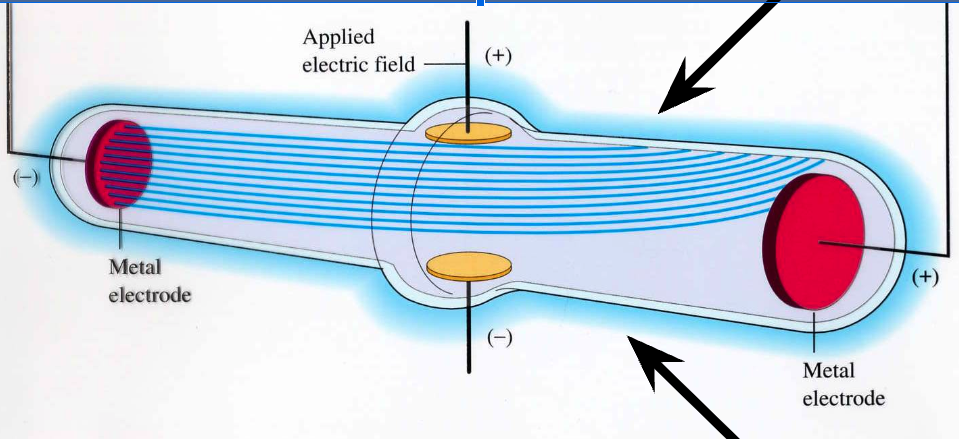
JJ Thomson
Discovered **Electrons (negative charge)** by using cathode ray tube.
3
New cards
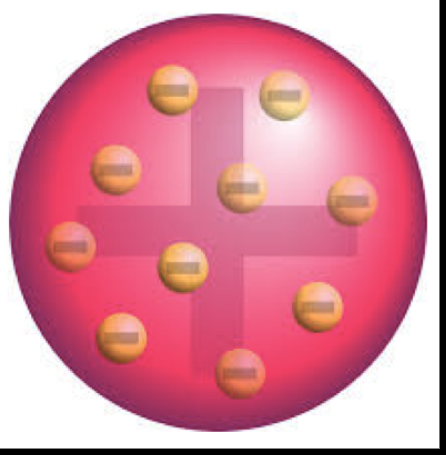
JJ Thomson Model
His model was Plum Pudding Model
4
New cards
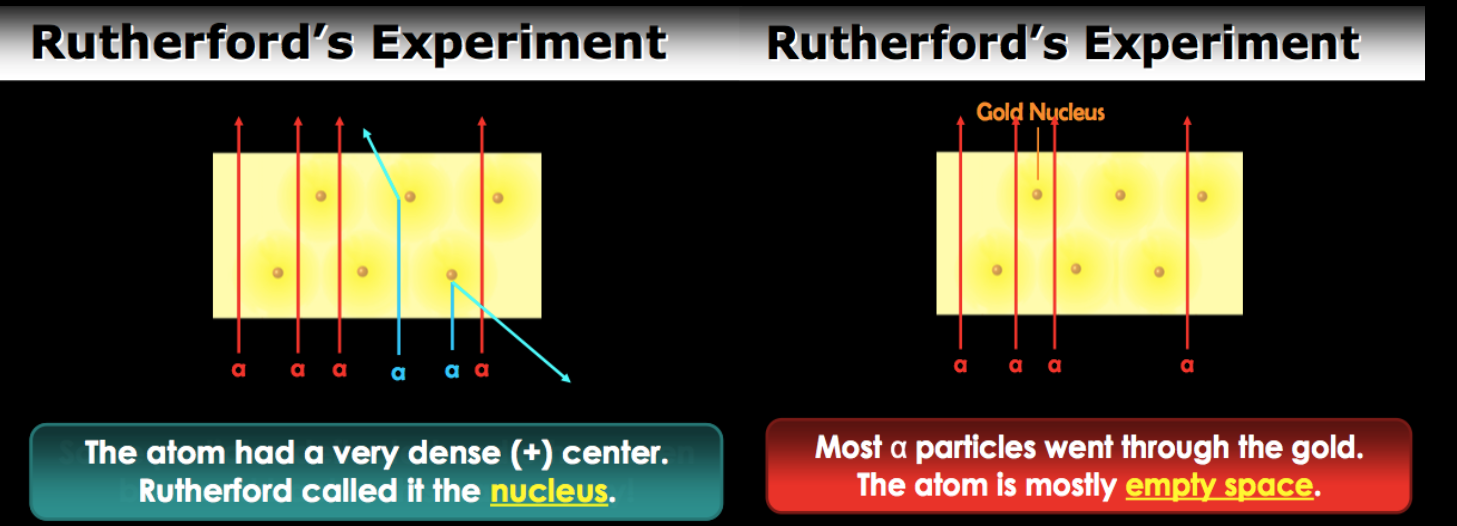
Rutherford
Discovered the nucleus and performed the gold foil experiment. Most of particles went through the gold so it shows atom is mostly empty. But some of them deflected and others bounced back. If the atom had very dense center and this when he discovered the nucleus.
5
New cards
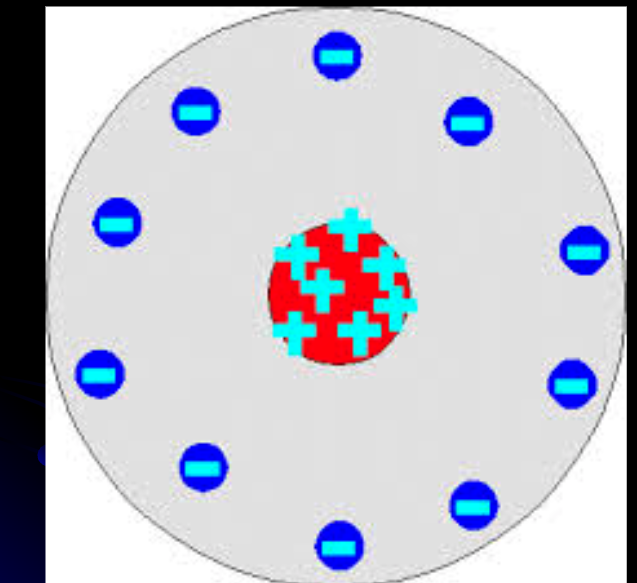
Rutherford Model
This what the model looks like
6
New cards
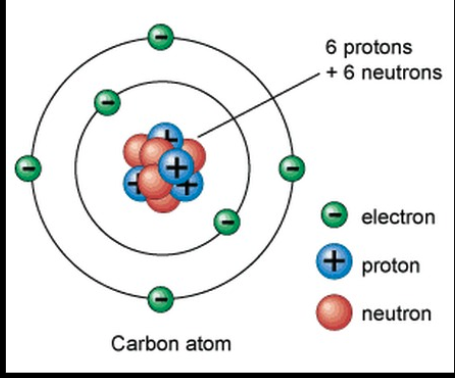
Niels Bohr (Bohr Model)
Electrons orbited the nucleus like planets do with sun. Also called the Planetary Model.
7
New cards
James Chadwick
Discovered Neutrons and discovered that they have no charge. Also neutrons are found in the nucleus.
8
New cards
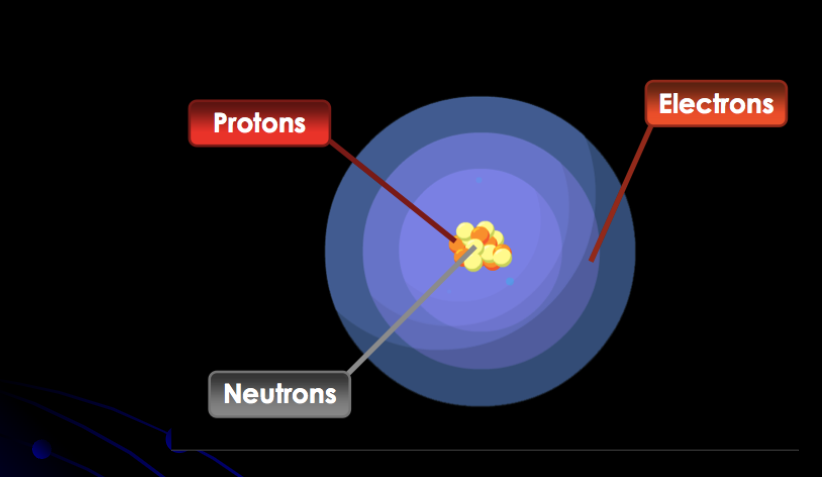
Erwin Schrödinger
Created the Wave Model and is which is the current model. Said electrons are found in electron cloud.
9
New cards
Heisenberg
Position and speed of a particle, such as electrons.
10
New cards
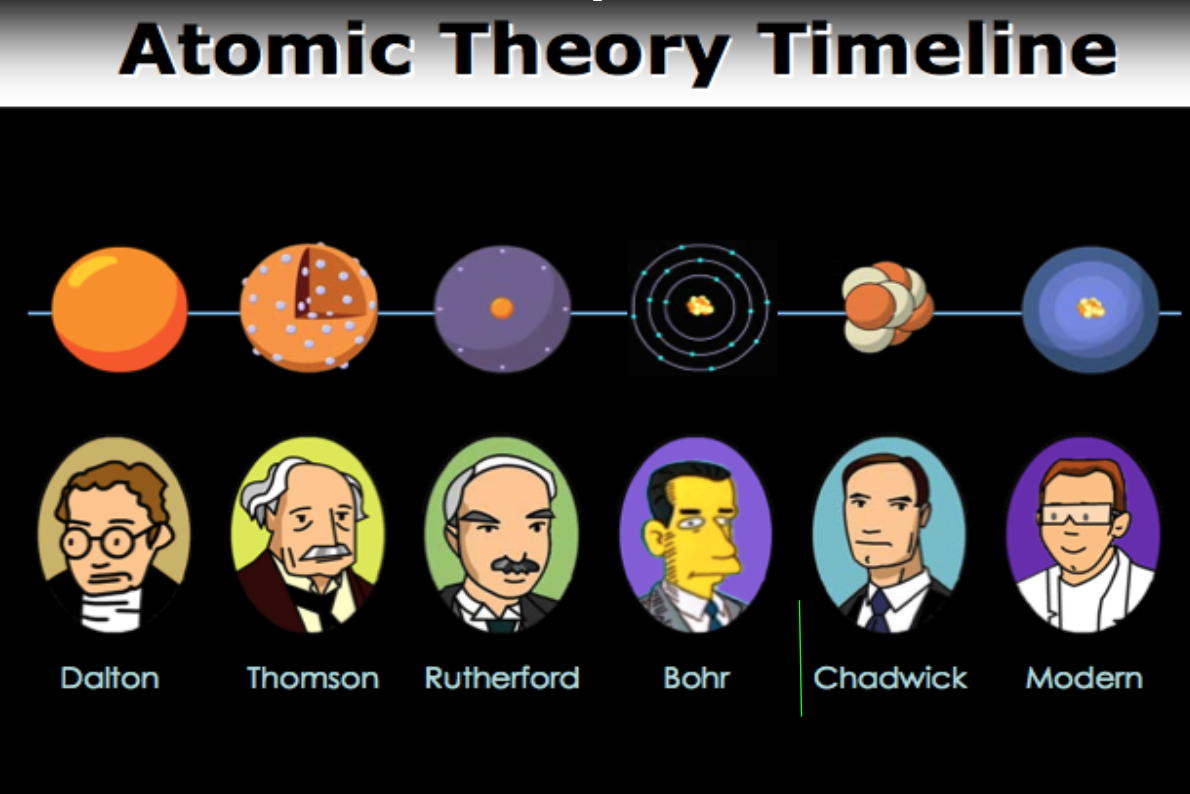
Timeline of Atomic Theory
Here’s the timeline of the atomic theory.