CHEM 2211- Test 2
0.0(0)
Card Sorting
1/106
There's no tags or description
Looks like no tags are added yet.
Last updated 3:37 PM on 7/6/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
1
New cards
2
New cards
Newman Projections
Dot within a circle, dot is the front carbon and circle is the back carbon
3
New cards
Two Types of Newman Projections
Eclipsed and staggered
4
New cards
Eclipsed Newman Projection
All groups overlap. Substituents on the front carbon overlap with substituents on the back carbon. These Newman projections have eclipsed energy interactions.
5
New cards
Eclipsed energy interactions
Interactions between molecules on the atom that are 60 degrees apart. Always 3 interactions. These interactions are less stable and have higher energy.
6
New cards
Staggered Newman Projections
All substituents on the front carbon are 60 degrees apart from substituents on the back carbon. This results in an upside down and right side up Y shape. These projections have gauche interactions.
7
New cards
Anti-staggered conformation
The largest substituent on the front carbon is 180 degrees apart from the largest substituent on the back carbon.
8
New cards
Gauche interactions
Non-hydrogen substituents 60 degrees away from each other interact. Hydrogen atoms will NEVER give rise to gauche interactions.
9
New cards
Shifting between Newman Projections
To shift between eclipsed and staggered rotate 60 degrees. To change between staggered and staggered or eclipse and eclipsed, rotate 120 degrees.
Anti staggered has no gauche interactions and therefore is the most stable Newman Projection.
Anti staggered has no gauche interactions and therefore is the most stable Newman Projection.
10
New cards
Drawing Newman Projections
1. Draw skeletal form of molecule
2. Identify front and back carbons
3. Draw Newman template (staggered or eclipsed)
4. Add substituents which are on the front carbon to the front carbon of the Newman template (dot)
5. Do the same with the back carbon
11
New cards
Drawing 2nd Newman Projection
1. Draw new Newman Template
2. Keep front carbon and its substituents still
3. Rotate back carbon and its substituents 60 degrees. Rotating 120 degrees interconverts staggered-staggered or eclipsed-eclipsed
12
New cards
Drawing 3rd Newman Projection
1. Draw a new Newman template and add substituents to their new positions.
2. Hold front carbon still and rotate back carbon 60 degrees.
3. If you started staggered your new projection should be staggered again or vice versa
4. There’s always 3 staggered and 3 eclipsed Newman projections for every compound.
13
New cards
Energy calculations for Newman Projections
1. Determine interactions (gauche or eclipsed)
2. Determine the type for each (ex. CH3-CH2CH3)
3. Find the interaction on a given table and determine its energy value
4. Sum all of the interactions to find the total energy associated with a given Newman.
5. Lowest energy=most stable
14
New cards
Chair conformation
The shape taken on by cyclohexanes.
15
New cards
Axial conformation
Substituents and hydrogens point straight up or straight down.
16
New cards
Equatorial conformation
Hydrogen and substituents are always slanted and point up and outward or down and outward.
17
New cards
General rules for equatorial and axial
There will always be one axial and one equatorial position for every carbon atom in a cyclohexane chair conformation. If axial is pointed up then equatorial must point down.
18
New cards
Chair flip
Rotating around the bonds in a cyclohexane chain. Far nose on left and dar nose on right will point in opposite directions of their original orientation. All substituents that are axial become equatorial and vice versa. The directions in which substituents point do not change (up or down).
19
New cards
Cyclohexane Energetics
Gauche and 1,3- Diaxial interactions
20
New cards
Gauche interactions
Between 2 non-hydrogen substituents that are attached to adjacent carbons. Both substituents are equatorial or one is equatorial and the other is axial. Cannot both be axial because that would make the substituents 180 degrees away from each other (one would point up and the other would point down).
21
New cards
1,3-Diaxial interactions
Takes place between axial substituents that are pointed in the same direction. Hydrogens can participate but 2 hydrogen atoms can never have a 1,3- Diaxial interaction.
22
New cards
Calculating Energy for Chair Conformations
1. Determine the interactions to find the associated energy
2. Add energy from all interactions for each chair
3. Chair with the greatest energy = least stable
4. least energy = most stable
5. No energy table? Look the chair with the bulkiest substituent in the equatorial position. Equatorial is more stable than axial.
23
New cards
Solving a chair problem from skeletal structure
1. Draw chair conformation and its corresponding flip template
2. In general : Wedge on skeletal chair means pointing up, dashed means pointing down. Unless there is a numbering system that has been given and are ONLY asked to draw the most stable conformation chair.
3. Number flat ring 1-6 based on nomenclature rules. Identify substituents on each carbon in the flat ring.
4. Number chair conformation and add substituents to it based on the numbering system.
5. Draw the chair flipped and number it relative to the original chair and its substituents.
24
New cards
Newmans and cyclic rings
The energetic interactions present on a chair conformation are present on a cyclic Newman conformation. (gauche and diaxial)
25
New cards
Strain energies
Steric strain, angle strain, torsional strain
26
New cards
Steric strain
The repulsion of neighboring electron clouds.
Gauche interactions are examples of this.
This strain increases with the size of interacting atoms or groups.
Gauche interactions are examples of this.
This strain increases with the size of interacting atoms or groups.
27
New cards
Angle strain
Heat of combustion is used to compare the angle strain of various cycloalkanes. The larger the heat of combustion, the more angle strain the ring has. Increases as the size of the ring deviates from the cyclohexane, with 3-membered rings having the highest angle strain.
28
New cards
Torsional strain
Arises from the stored potential energy within bonds.
Eclipsing interactions are an example.
If we assumed that all cycloalkanes were planar, we would see that as cycloalkane increases in size, the number of eclipsing interactions would increase and so would torsional strain.
Eclipsing interactions are an example.
If we assumed that all cycloalkanes were planar, we would see that as cycloalkane increases in size, the number of eclipsing interactions would increase and so would torsional strain.
29
New cards
Naming compounds from a Newman projection or chair conformation
1. Convert compound to skeletal structure
2. For Newman, identify the longest chain on the front carbon and count from there through the back carbon. The numbering is not necessarily the parent chain but it will help keep track of all the atoms. Once it has been translated to skeletal, the numbering of the parent chain will be reevaluated
3. Name compound in accordance with the rules from part 1.
4. If given a disubstituted chair conformation, do not forget cis or trans.
30
New cards
Functional groups
Collection of atoms bound together in a specific manner
31
New cards
Alkane
Carbon singly bonded to a carbon
32
New cards
Alkene
Carbon double bound to a carbon
33
New cards
Alkyne
Carbon triple bound to a carbon
34
New cards
Alkyl Halide
Carbon bound to a halogen
35
New cards
Ether
A carbon-oxygen-carbon bond
36
New cards
Alcohol
Carbon bound to an OH group
37
New cards
Amine
Nitrogen bound to a hydrogen or carbon atoms
38
New cards
Nitrile
Carbon triple bound to a Nitrogen
39
New cards
Thiol
Carbon bound to a sulfur
40
New cards
Arene
Cyclic ring of alternating carbon double bonds.
41
New cards
Carbonyl-Containing functional groups
Carbonyl: Carbon-oxygen double bond
42
New cards
Carboxylic Acid
Carbonyl carbon with an OH attached to one side
43
New cards
Ester
Carbonyl with a carbon on one side and the oxygen is attached to more carbons
44
New cards
Aldehyde
Carbonyl is attached to hydrogen on one side and carbon on the other.
45
New cards
Ketone
Carbonyl is attached to carbons on either side
46
New cards
Amide
Carbonyl carbon is attached to nitrogen on one side and a carbon on the other (nitrogen can be attached to two hydrogens or a carbon)
47
New cards
Acid Halide
Carbonyl carbon bound to halogen on one side, carbon on the other
48
New cards
Anhydride
Two carbonyls connected via an oxygen
49
New cards
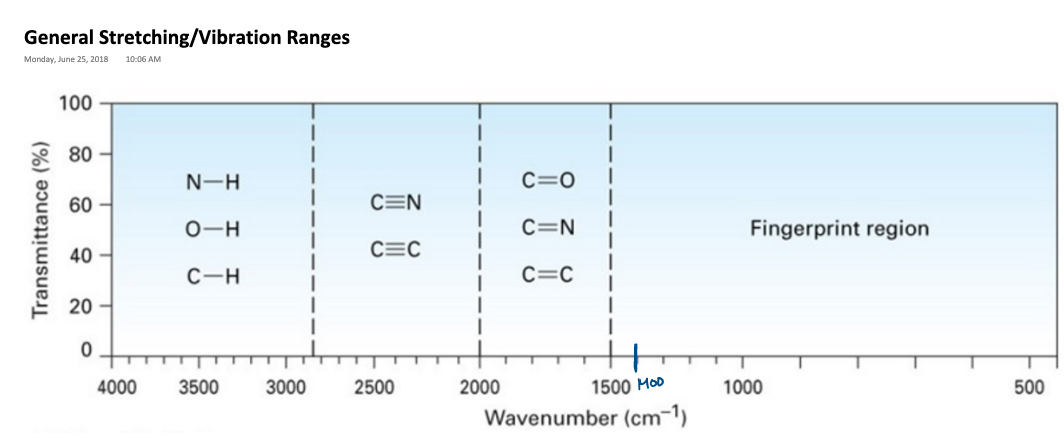
Infrared Spectroscopy
* Uses infrared radiation to excite the vibrational states of organic compounds, which gives information regarding what functional groups are present on a particular compound.
* It is QUALITATIVE- can not tell about the quantity of the functional groups, cannot tell us information about the formula.
* Can only tell us the presence or absence of a functional group
* Specific groups exhibit absorbance peaks (stretch) in specific ranges of the infrared spectrum.
\
* It is QUALITATIVE- can not tell about the quantity of the functional groups, cannot tell us information about the formula.
* Can only tell us the presence or absence of a functional group
* Specific groups exhibit absorbance peaks (stretch) in specific ranges of the infrared spectrum.
\
50
New cards
Interpreting the IR spectrum
* There are two line drawn at the spectrum at 3000 cm ^-1 and 1500 cm^-1. The line at 3000cm ^-1 separates aliphatic hydrogen stretches from vinylic hydrogen stretches.
* Aliphatic hydrogen-carbon stretches appear to the right to the line at 3000cm ^-1 while vinylic appear to the left of that line.
* The line at 1500 cm^-1 separates the fingerprint region (0-1500cm^-1) called this because it is the fingerprint of the organic molecule. We do not tend to focus on the fingerprint region because it contains the stretches corresponding to all the single bonds in the molecule.
* Aliphatic hydrogen-carbon stretches appear to the right to the line at 3000cm ^-1 while vinylic appear to the left of that line.
* The line at 1500 cm^-1 separates the fingerprint region (0-1500cm^-1) called this because it is the fingerprint of the organic molecule. We do not tend to focus on the fingerprint region because it contains the stretches corresponding to all the single bonds in the molecule.
51
New cards
Aliphatic C-H stretch
sp3 carbons bound to hydrogen
52
New cards
Vinylic C-H stretch
sp2 carbon hydrogen stretches
53
New cards
Alkyne C-H stretch
sp carbon hydrogen bond
54
New cards
Alcohol O-H stretch
Bond between Oxygen and Hydrogen
55
New cards
N-H stretch
Bond between nitrogen and hydrogen
56
New cards
Terminal alkynes
Located at the very end of a molecule.
End with a hydrogen that can be seen on the IR spectrum.
End with a hydrogen that can be seen on the IR spectrum.
57
New cards
Internal Alkynes
Located in the middle of molecules. Not attached to hydrogens. Have no alkyne C-H stretch. Instead, have C-triple bond- C stretch
58
New cards
Alkane/Aliphatic (C-H sp3) stretch
Stretch occurs at 2850-2960 cm (toward the right of 3000)
Slightly jagged shape on its own but its shape can change depending on the other stretches present in the IR.
Slightly jagged shape on its own but its shape can change depending on the other stretches present in the IR.
59
New cards
Vinylic C-H (sp2) stretch
* Occurs between 3020-3100 cm
* Not too deep, jagged
* Not too deep, jagged
60
New cards
Alkene C-double bond- C stretch
* Occurs at 1640-1680
* Pointy Voldemort finger
* Pointy Voldemort finger
61
New cards
Arene peak
* Occurs 1450- 1600
* Also very thin and pointy
* Also very thin and pointy
62
New cards
Arene undertones
* Occur 1660-2000
* Can be indicative of an arene peak
* Jagged and shallow, resemble shark teeth
* Can be indicative of an arene peak
* Jagged and shallow, resemble shark teeth
63
New cards
Alkyne stretches (C-H and C-triple bond-C)
* C-H stretch at 3300
* C-triple bond-C stretch occurs at 2100-2260
* C-triple bond-C stretch occurs at 2100-2260
64
New cards
Alcohol stretch
* The most obvious of the stretches and what you should typically look for first.
* Occurs at 3200-3650
* Resembles a fat finger, broad and strong peak
* Occurs at 3200-3650
* Resembles a fat finger, broad and strong peak
65
New cards
Ether stretch (C-O)
* At 1050-1250
* The only peak to look for in the fingerprint region
* Deep, curvy stretch
* The only peak to look for in the fingerprint region
* Deep, curvy stretch
66
New cards
Amine stretch (N-H stretch and N-H bend)
* N-H stretch occurs at 3400-3650
* Broad, strong, slightly jagged and a bit shallow. Often has substructure indicative of amine substitution
* N-H bend: 1600cm
* Broad, strong, slightly jagged and a bit shallow. Often has substructure indicative of amine substitution
* N-H bend: 1600cm
67
New cards
Aldehyde stretch (C-H)
* 2820-2695
* There is a transmittance of two peaks (resemble fangs)
* Has a carbonyl(C-double bond-O) stretch at 1670-1780
* There is a transmittance of two peaks (resemble fangs)
* Has a carbonyl(C-double bond-O) stretch at 1670-1780
68
New cards
Ketone stretches
* Aliphatic stretch (2850-2960)
* Carbonyl stretch (1670-1780)
* Carbonyl stretch (1670-1780)
69
New cards
Ester stretch
* Aliphatic C-H (2850-2960)
* C-O stretch: (1050-1250) fingerprint region
* Carbonyl C-double bond-O 1670-1780
* C-O stretch: (1050-1250) fingerprint region
* Carbonyl C-double bond-O 1670-1780
70
New cards
Carboxylic acid
* Aliphatic C-H stretch: 2850-2960
* Carboxylic acid O-H: 3500-3000 (broad and very strong, bleeds into C-H sp2)
* Carbonyl C-double bond-O: 1670-1780
* Carboxylic acid O-H: 3500-3000 (broad and very strong, bleeds into C-H sp2)
* Carbonyl C-double bond-O: 1670-1780
71
New cards
Nitrile
* Aliphatic C-H stretch- 2850-2960
* Nitrile C- triple bond- N: 2100-2260 (similar to alkyne)
* Thin Voldemort finger, shallow
* Nitrile C- triple bond- N: 2100-2260 (similar to alkyne)
* Thin Voldemort finger, shallow
72
New cards
Memorizing IR
\
73
New cards
Mass Spectrometry
Way to weigh an unknown sample on a molecular level to determine its molecular weight. Molecular formula can be determined from the weight.
* Sample is injected into MS, sample is ionized by a beam of electrons
* Following ionization, the charged sample is compelled toward a mass analyzer (magnet). As the sample flows through the mass analyzer, it can remain intact or fragment into smaller pieces.
* Sample is injected into MS, sample is ionized by a beam of electrons
* Following ionization, the charged sample is compelled toward a mass analyzer (magnet). As the sample flows through the mass analyzer, it can remain intact or fragment into smaller pieces.
74
New cards
Mass analyzer
* Maas analyzer sorts out various types of ions generated from the ionization and fragmentation process. Only cations can enter the mass analyzer.
* After ions are sorted by the mass analyzer the ions smash into a detector that provides a signal (spectral peak) corresponding to the mass of each sorted ion. The sorted ions can be visualized on a graph known as mass spectrum
* The magnet on the mass analyzer is negatively charged.
* After ions are sorted by the mass analyzer the ions smash into a detector that provides a signal (spectral peak) corresponding to the mass of each sorted ion. The sorted ions can be visualized on a graph known as mass spectrum
* The magnet on the mass analyzer is negatively charged.
75
New cards
Mass Spec Rules
Nitrogen rule: If the molecular ion peak is odd then there is at least one nitrogen atom present in the molecular formula of the sample
Bromine Rule: Carbon isotopes are generally ignored but for bromine if there is an isotope that is M+2 (2 mass units away from the molecular peak)a and has relatively the same intensity as the peak hen that means there is bromine present in your molecular formula.
Chlorine rule: M+2 peak but one-third the intensity of the M+ peak
Bromine Rule: Carbon isotopes are generally ignored but for bromine if there is an isotope that is M+2 (2 mass units away from the molecular peak)a and has relatively the same intensity as the peak hen that means there is bromine present in your molecular formula.
Chlorine rule: M+2 peak but one-third the intensity of the M+ peak
76
New cards
Determining Molecular Formulas for compunds
* Look at mass spectrum for Nitrogen, Chlorine, and Bromine
* Look at IR for oxygen
* Subtract heteroatoms from M+ to find the mass of the sample that only contains hydrogen and carbon atoms
* Rule of 13: Divide the number calculated by 13, (The molar mass of carbon +1) Gives us the maximum number of carbons that can be present in the molecular formula. Round down
* Solve for hydrogens: Equal to the remainder of the mass. Multiply the number of carbons found from rule of thirteen by 12, add the weight of the heteroatoms. Subtract this from the value on the molecular ion peak.
* Look at IR for oxygen
* Subtract heteroatoms from M+ to find the mass of the sample that only contains hydrogen and carbon atoms
* Rule of 13: Divide the number calculated by 13, (The molar mass of carbon +1) Gives us the maximum number of carbons that can be present in the molecular formula. Round down
* Solve for hydrogens: Equal to the remainder of the mass. Multiply the number of carbons found from rule of thirteen by 12, add the weight of the heteroatoms. Subtract this from the value on the molecular ion peak.
77
New cards
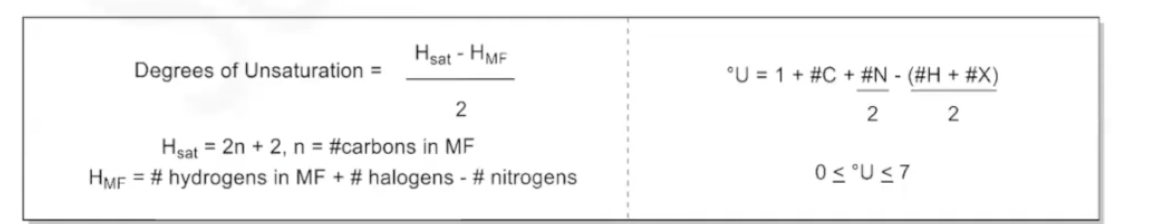
Degrees of Unsaturation
* used to check if the molecular formula found is reasonable
* If the degree of unsaturation is less than 0 or greater than 7 then the formula is unreasonable
* If it is less than 0, increase the carbon count by 1 and recalculate.
* If it is more than 7, decrease carbon count by 1.
* The degrees of unsaturation will tell us how many cyclic rings or pi bonds are present in a compound based on its molecular formula.
* 1 degree: One ring or one double bond
* 2 Degrees: Two rings, two double bonds, a ring and a double bond, or a triple bond
* 3: Some combination of rings, double bonds, or triple bonds.
* If the degree of unsaturation is less than 0 or greater than 7 then the formula is unreasonable
* If it is less than 0, increase the carbon count by 1 and recalculate.
* If it is more than 7, decrease carbon count by 1.
* The degrees of unsaturation will tell us how many cyclic rings or pi bonds are present in a compound based on its molecular formula.
* 1 degree: One ring or one double bond
* 2 Degrees: Two rings, two double bonds, a ring and a double bond, or a triple bond
* 3: Some combination of rings, double bonds, or triple bonds.
78
New cards
Two types of cleavages
* Homolytic
* Heterolytic
\
* Heterolytic
\
79
New cards
Homolytic
Breaking bonds between two atoms of similar connectivity
80
New cards
Heterolytic
Breaking a bond between two atoms of different electronegativity.
81
New cards
Alkane fragmentation
\
* Undergo homolytic cleavage
* Form one radical fragment and a cation
* Neutral fragments can be seen as losses of mass from the molecular ion peak, find these by calculating the difference between the molecular ion peak and the fragmentation peaks.
* Fragments will show up at a mass to charge that corresponds to the mass
* Some common ones:
Methyl: 15m/z
Ethyl: 29m/z
Propyl: 43m/z
Butyl: 57m/z
More stable carbocation= more intense peaks
* Tertiary is the most stable then secondary then primary, then methyl
* Undergo homolytic cleavage
* Form one radical fragment and a cation
* Neutral fragments can be seen as losses of mass from the molecular ion peak, find these by calculating the difference between the molecular ion peak and the fragmentation peaks.
* Fragments will show up at a mass to charge that corresponds to the mass
* Some common ones:
Methyl: 15m/z
Ethyl: 29m/z
Propyl: 43m/z
Butyl: 57m/z
More stable carbocation= more intense peaks
* Tertiary is the most stable then secondary then primary, then methyl
82
New cards
Alcohol Fragmentation
* Undergo homolytic alpha cleavage and heterolytic dehydration cleavage.
* Alpha cleavage is the cleavage between alpha and beta carbons. Alpha carbon is the carbon to which the hydroxyl group is attached. Beta carbon is the carbon next door to the alpha carbon.
* Primary alcohols will always undergo one alpha cleavage to form a fragment which has m/z of 31.
* Secondary alcohols undergo two alpha cleavages
* Alpha cleavage is the cleavage between alpha and beta carbons. Alpha carbon is the carbon to which the hydroxyl group is attached. Beta carbon is the carbon next door to the alpha carbon.
* Primary alcohols will always undergo one alpha cleavage to form a fragment which has m/z of 31.
* Secondary alcohols undergo two alpha cleavages
83
New cards
Dehydration
The removal of a gamma hydrogen by the hydroxyl oxygen. The bond between the hydroxyl oxygen and the alpha carbon is then broken, forming water and a radical cation. Dehydration results in the loss of 18 m/z
84
New cards
Fragmentation of Alkyl Halides
Undergo homolytic alpha cleavage and heterolytic “halogen-leaving” cleavage.
Alpha Cleavage: Alpha carbon in halide is the carbon in which the halide group is directly attached.
primary halide-1 alpha cleavage
secondary- 2 alpha cleavages
tertiary- three alpha cleavages
Halogen leaving cleavage- The halogen laves with no charge, 1 double headed arrow (preferred cleavage)
Alpha Cleavage: Alpha carbon in halide is the carbon in which the halide group is directly attached.
primary halide-1 alpha cleavage
secondary- 2 alpha cleavages
tertiary- three alpha cleavages
Halogen leaving cleavage- The halogen laves with no charge, 1 double headed arrow (preferred cleavage)
85
New cards
Fragmentation of Ethers and Amines
Alpha cleavage- Cleavage between the alpha carbon and the beta carbon alpha is the carbon in which the oxygen/amine is directly attached.
86
New cards
Fragmentation of Aldehydes and Ketones
Alpha cleavage- corresponds to the bond cleavage between ipso carbon and alpha carbon. Ipso carbon is the carbonyl carbon, alpha is connected to the ipso.
McClafferty Rearrangement: takes place through the cleavage between alpha and beta carbons. A gamma hydrogen is required for the McClafferty Rearrangement to take place. Gamma hydrogen atoms correspond to the hydrogen atoms attached to the gamma carbon.
McClafferty Rearrangement: takes place through the cleavage between alpha and beta carbons. A gamma hydrogen is required for the McClafferty Rearrangement to take place. Gamma hydrogen atoms correspond to the hydrogen atoms attached to the gamma carbon.
87
New cards
Solving a Structure Using Mass Spectroscopy
88
New cards
Chirality
Any atom within a compound which has bonds to four different substituents. Hydrogens and lone pairs of electrons should be considered to be substituents.
89
New cards
Typical atoms that can have chirality
* Carbon (must b sp3)
* Sulfur
* Phosphorus
* \*Nitrogen( can be chiral due to its lone pair of electrons however, chiral nitrogens can undergo spontaneous inversion, will not rotate plane polarized light).
* Sulfur
* Phosphorus
* \*Nitrogen( can be chiral due to its lone pair of electrons however, chiral nitrogens can undergo spontaneous inversion, will not rotate plane polarized light).
90
New cards
Typical atoms that will never have chirality
Oxygen
Halogens
Hydrogen
Halogens
Hydrogen
91
New cards
Determining Chirality
To determine chirality, there must be 3-D information given (Wedges and dashes vs. straight lines
Exceptions: Fisher Projections, Newman Projections and Cyclohexane Chair Conformations (stereochemistry can still be determined regardless if only straight lines are given)
Assign Priority: Assign priority to each of the atoms attached to the chiral center based on atomic mass of the atom directly attached. Highest priority will receive a 1 and lowest will receive a 4.
If the lowest substituent is in the back (dashed), move to the next step, if not switch the lowest priority substituent with the one that is currently in the back.
Draw directionality arrows Ignore the lowest priority substituent and draw arrows from 1 to 2, 2 to 3, and 3 back to one.
Exceptions: Fisher Projections, Newman Projections and Cyclohexane Chair Conformations (stereochemistry can still be determined regardless if only straight lines are given)
Assign Priority: Assign priority to each of the atoms attached to the chiral center based on atomic mass of the atom directly attached. Highest priority will receive a 1 and lowest will receive a 4.
If the lowest substituent is in the back (dashed), move to the next step, if not switch the lowest priority substituent with the one that is currently in the back.
Draw directionality arrows Ignore the lowest priority substituent and draw arrows from 1 to 2, 2 to 3, and 3 back to one.
92
New cards
R-Stereochemistry
Corresponds to a chiral center with directionality arrows that move clockwise.
* If you had to switch lowest priority to the back, then you are determining the stereochemistry of the switched compound.
* Stereochemistry of the original compound will be opposite the stereochemistry determined for the switched one.
* If you had to switch lowest priority to the back, then you are determining the stereochemistry of the switched compound.
* Stereochemistry of the original compound will be opposite the stereochemistry determined for the switched one.
93
New cards
S-Stereochemistry
Corresponds to the chiral center in which the directionality arrows are moving counter clockwise.
* If you had to switch lowest priority to the back, then you are determining the stereochemistry of the switched compound.
* Stereochemistry of the original compound will be opposite the stereochemistry determined for the switched one.
* If you had to switch lowest priority to the back, then you are determining the stereochemistry of the switched compound.
* Stereochemistry of the original compound will be opposite the stereochemistry determined for the switched one.
94
New cards
Prioritizing Larger Substituents
If two identical atoms are attached to the chiral center but are attached to different substituents, the first larger difference in the atomic number of the atoms they are attached to will be what we look for to determine which takes priority.
95
New cards
Prioritizing Substituents Making Pi Bonds
* If atoms that are making double or triple bonds are attache to the chiral center:
* assume all sigma bonds are real
* Assume each pi bond in a double or triple bond is making a fake bond to a fake atom of the same type as the one being made in the sigma bond of the double or triple bond.
* __Fake atoms beat atoms of lower atomic number but do not beat real atoms of the same type.__ If deciding between 3 real carbons, vs three fakes, the shell is tied and you move out again.
* assume all sigma bonds are real
* Assume each pi bond in a double or triple bond is making a fake bond to a fake atom of the same type as the one being made in the sigma bond of the double or triple bond.
* __Fake atoms beat atoms of lower atomic number but do not beat real atoms of the same type.__ If deciding between 3 real carbons, vs three fakes, the shell is tied and you move out again.
96
New cards
Meso Compound
A chiral compound with a plane of symmetry or “built in enantiomer”. Must have 2 chiral centers to be meso. Chiral centers are connected to the same substituents but have opposing chirality. (R and S)
97
New cards
Stereochemistry in Double bonds use E and Z or cis and trans
* Cis/Trans: Can only be used where an alkene has two opposing sp2 carbons with two identical substituents.
* E/Z: Draw a dotted line perpendicular to the double bond :
Determine which of the two groups attached takes priority on the right and then determine for the left. If the ones that take priority are on opposite sides then you have E stereochemistry if they are on the same side then it is Z stereochemistry.
* E/Z: Draw a dotted line perpendicular to the double bond :
Determine which of the two groups attached takes priority on the right and then determine for the left. If the ones that take priority are on opposite sides then you have E stereochemistry if they are on the same side then it is Z stereochemistry.
98
New cards
Stereoisomers
Set of compounds which have the same molecular formula, same atomic connectivity, but different orientations of substituents in space.
99
New cards
Enantiomers
Stereoisomers which are mirror images of one another, stereoisomers which have complete opposite centers of chirality.
100
New cards
Diastereoisomers
Stereoisomers which have two or more centers of chirality and one or more centers of chirality are opposite but not all. \*If all centers are opposite then it would be an enantiomer. E/Z isomers are considered diastereoisomers.