Bhavna Biol 304 Chpt 11 - Carbohydrates & Glycoproteins
1/201
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
202 Terms
Define a carbohydrate/What are carbohydrates primarily composed of?
carbon based molecules with lots of OH groups (can have additional groups/modifications)
What is the empirical formula for carbohydrates?
(CH2O)n
What is another name for carbohydrates?
Polyhydroxy Aldehydes & Ketones (and their derivatives)
Define monosaccharides
simplest carbohydrates that are aldehydes or ketones & contain 2 or > hydroxyl groups.
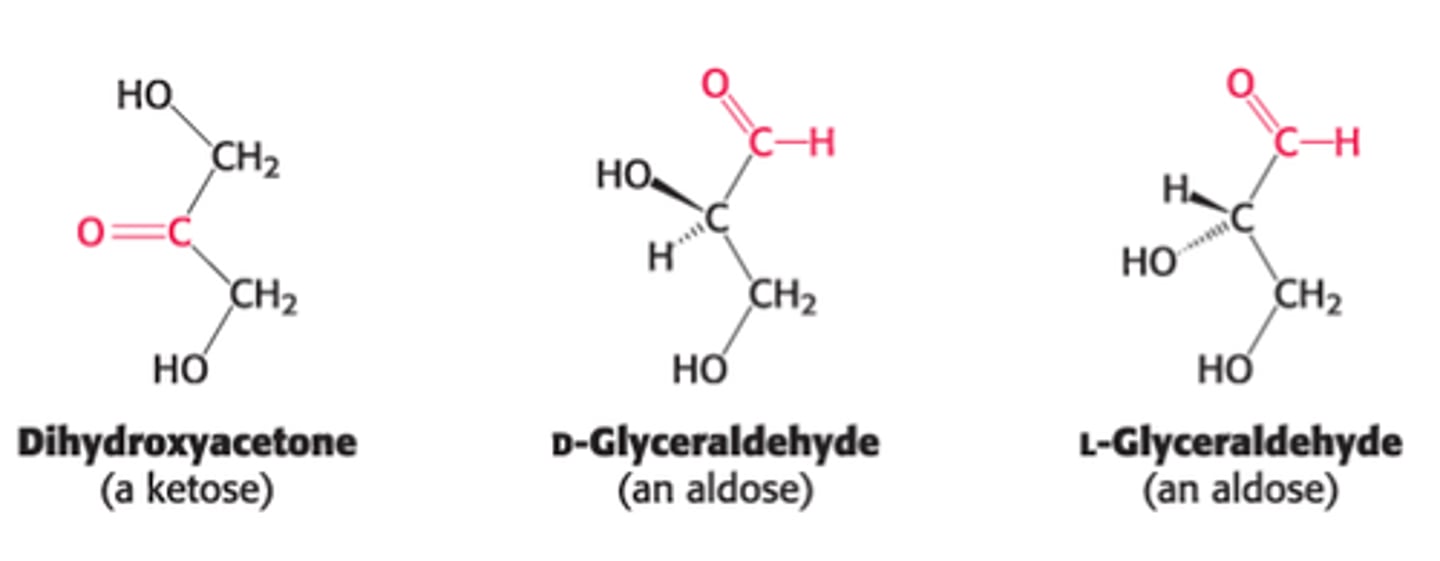
How many carbons can monosaccharides contain?
3-7 carbons in length where n = 3-7 in this empirical formula: (CH2O)n
What's another name for monosachharides?
simple sugars
What is the nomenclature of a monosaccharide based on?
length of the carbon chain & identity of the most oxidized group
Ketone vs Aldehyde
Give the nomenclature for 3C, 4C, 5C, 6C, 7C, keto group & aldehyde group
3C - Triose
4C - Tetrose
5C - Pentose
6C - Hexose
7C - Heptose
Keto Group - Ketose (most oxidized group is ketone)
Aldehyde Group - Aldose (most oxidized group is aldehyde)
Define molecular formula & structural formula
Molecular Formula - # and type of atoms
Structural Formula - arrangement of atoms
Define an isomer
molecules with the same molecular formula but different structural formula
What are the two main types of isomers in carbohydrates?
Constitutional isomers and Stereoisomers
Define constitutional isomers/structural isomers
molecules with identical molecular formulas but differ in how the atoms are ordered/diff skeletons or diff functional groups
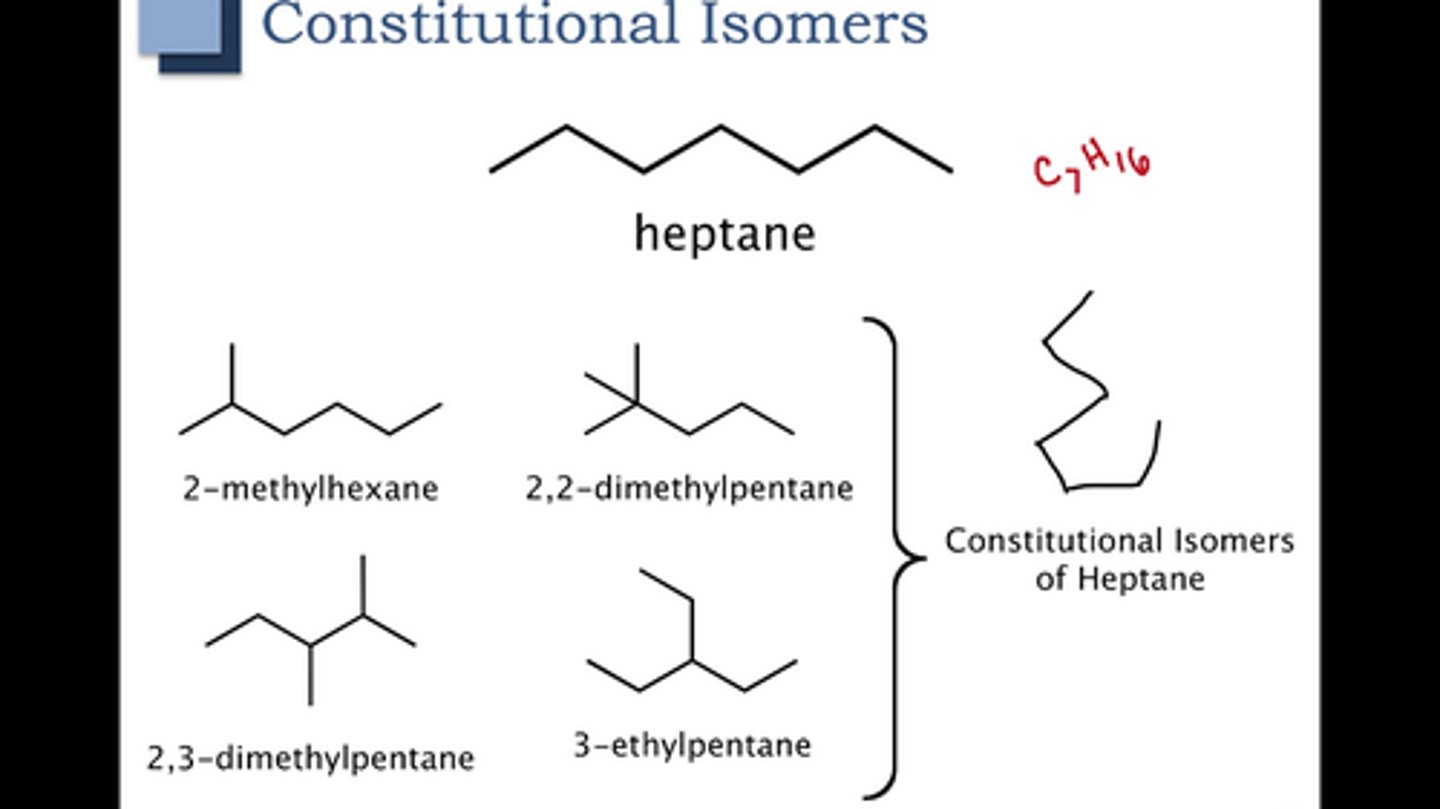
Define stereoisomers
molecules with same molecular formula & same bonding order but the atoms differ in spatial arrangement in space

Stereoisomers have which 2 configurations?
D or L, which is determined by the config of the asymmetric C atom farthest from the aldehyde or ketone group
Most vertebrae monosaccharides are in which config?
D config
Stereoisomers can be _______ or _______
Enantiomers - mirror images of e/o
Diastereoisomers - NOT mirror images of e/o, differ a chiral centers
Monosaccharides that have >3 C atoms and multiple asymmetric C's can exist as either or.

What's the formula for the # of possible stereoisomers?
2^n where n = # of asymmetric C's
Ex: For a 6C aldose with 4 asymmetric C atoms, there are 2^4 = 16 possible stereoisomers
Is an epimer a monosaccharide?
Yes
Define an epimer
Sugars that are diastereoisomers differing in configuration at a single asymmetric center
Ex: D-Glucose & D-Mannose are epimeric at C2. D-Glucose & D-Galactose are epimeric at C4.
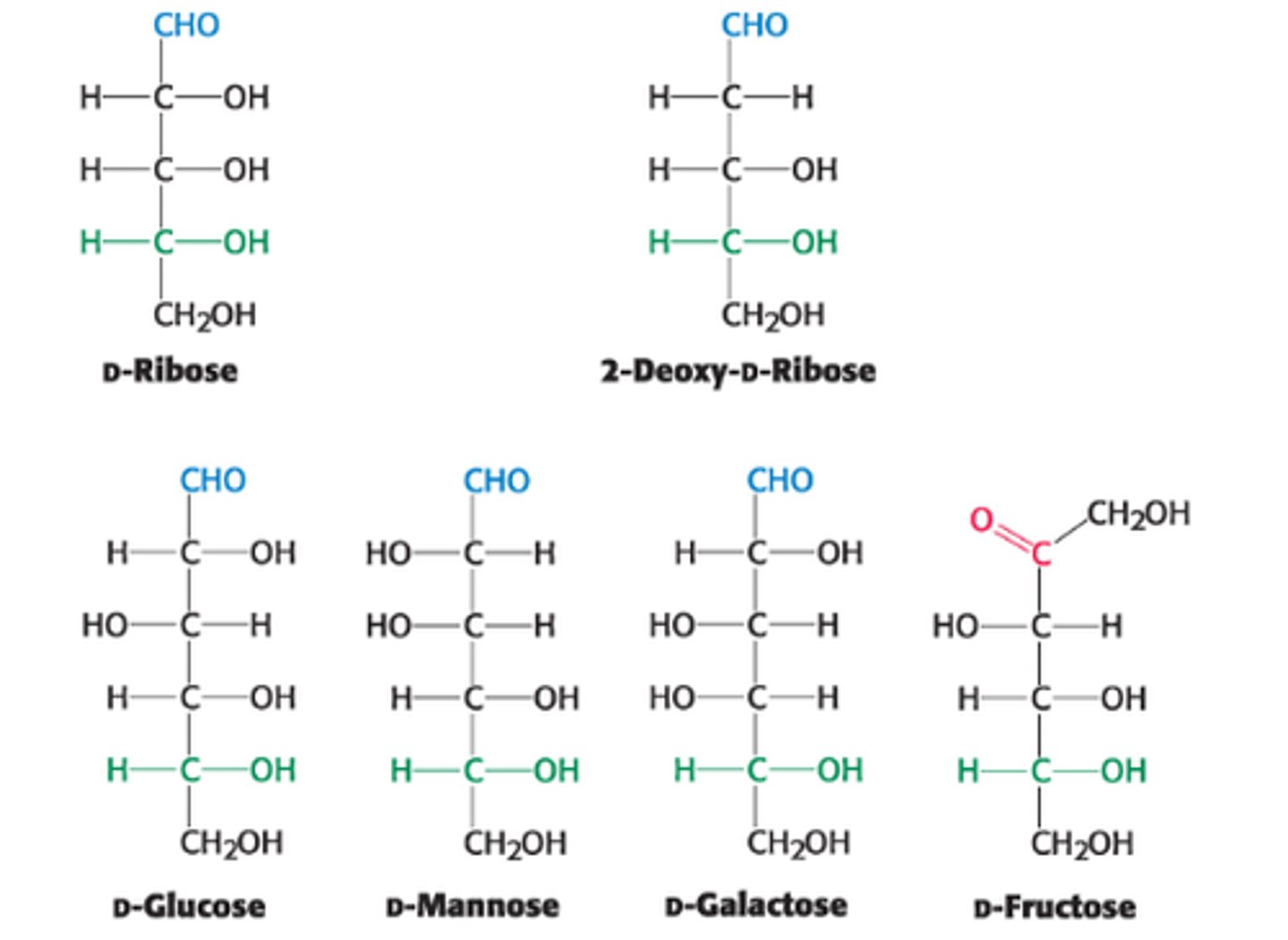
Ketoses have 1 less _______ than aldoses with the same # of C atoms
asymmetric C
What is the predominant form of monosaccharides?
ring formation where the open chain forms of these sugars cyclize into rings
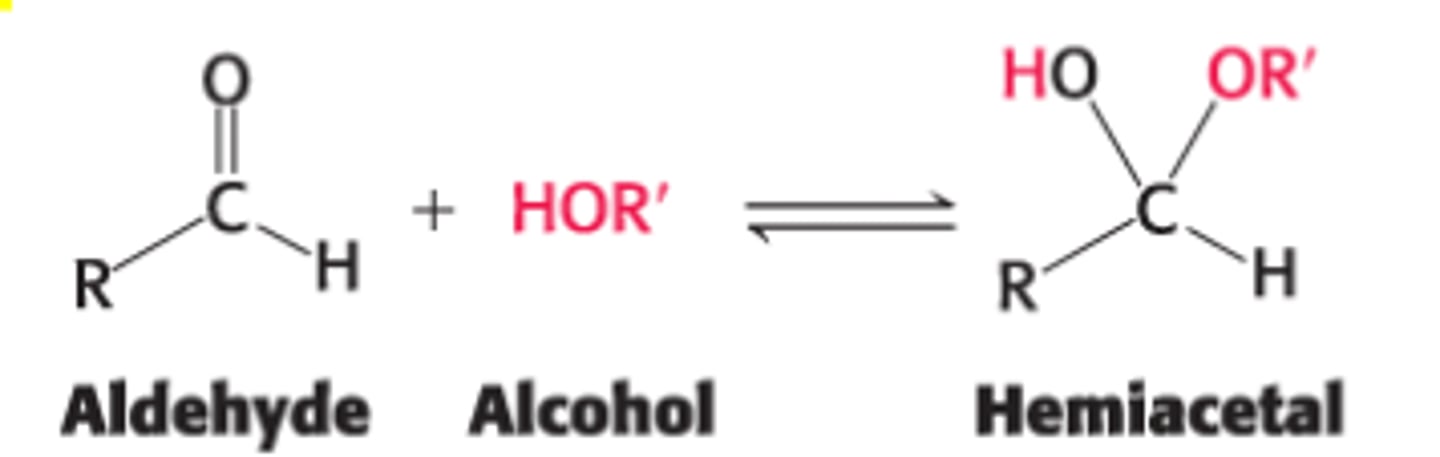
Define a hemiacetal
aldehyde + alcohol = hemiacetal
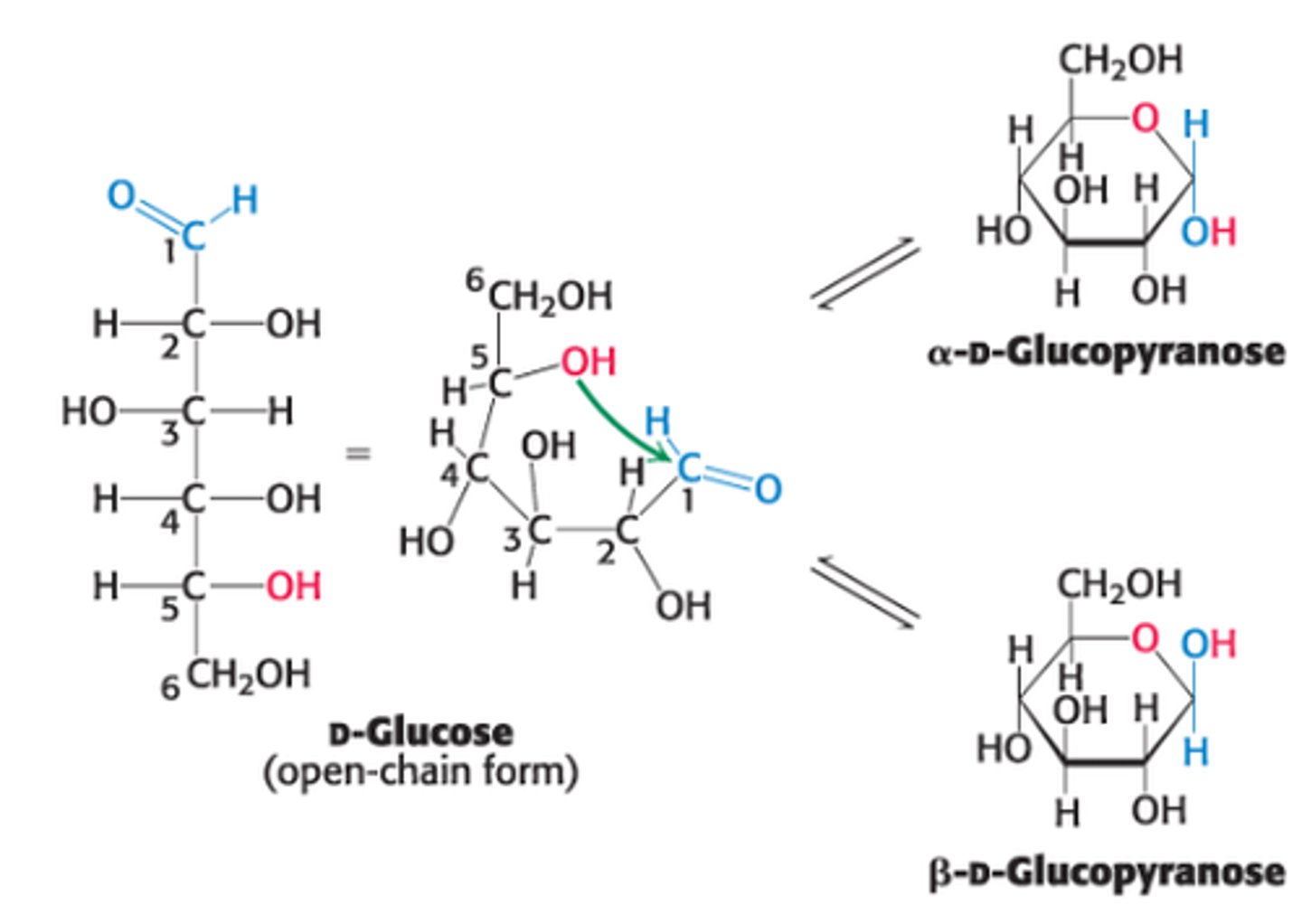
Explain the formation of intramolecular hemiacetal in open-chain form of glucose
ring that forms when C5 OH group attacks O atom of C1 aldehyde group in the open-chain form of glucose
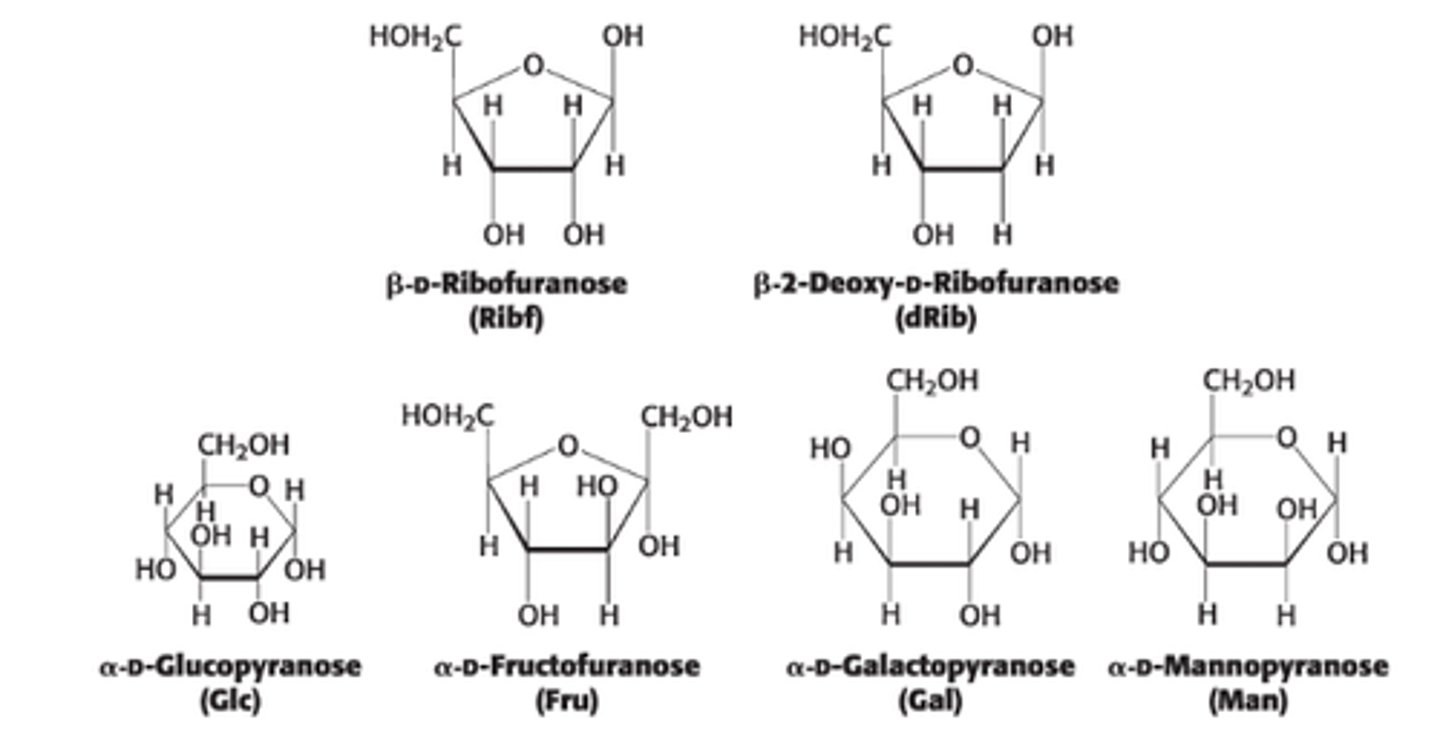
What 2 anomeric forms can result from pyranose formation?
𝛼 & β are both in rapid equilibrium with the open-chain/linear form
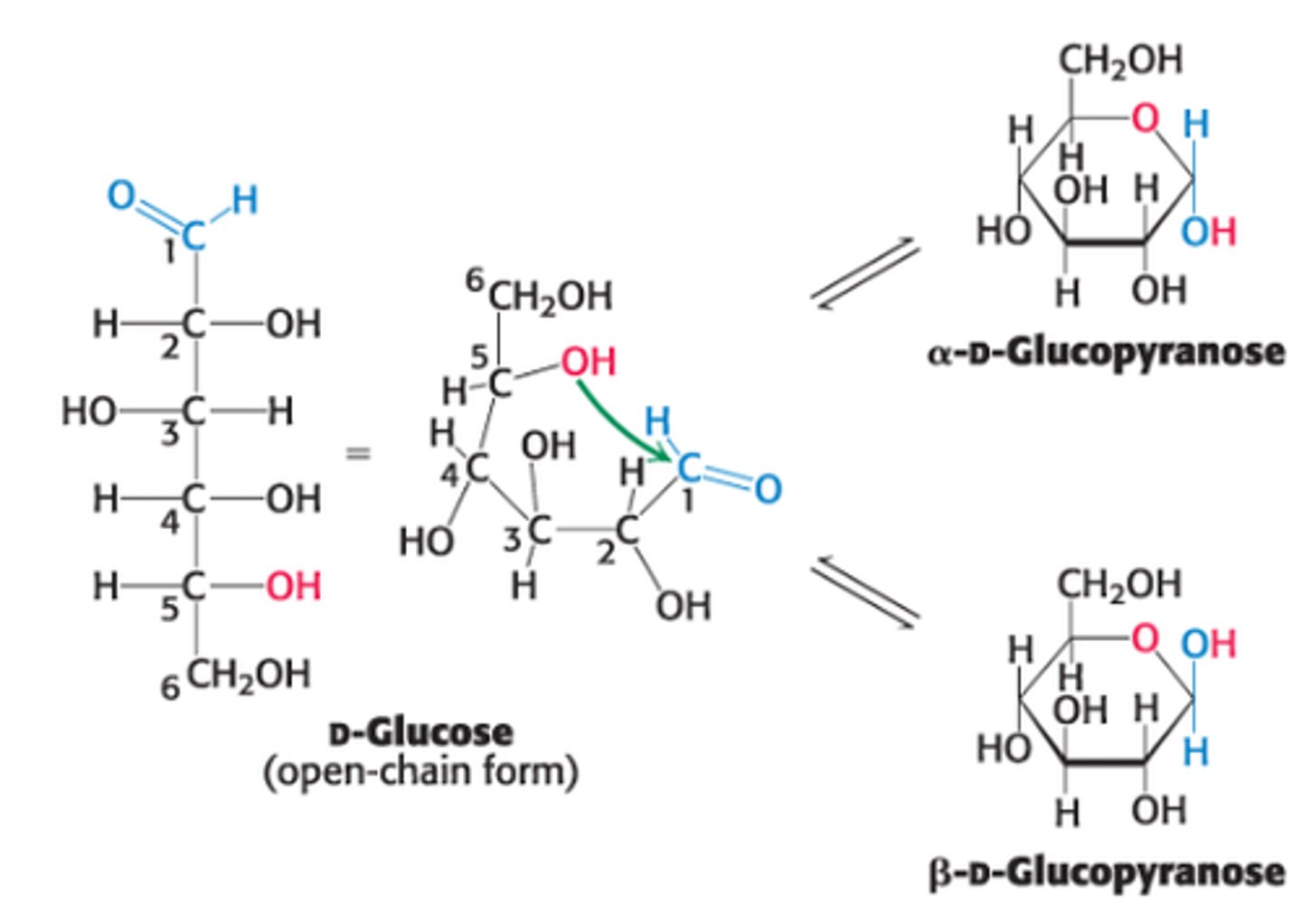
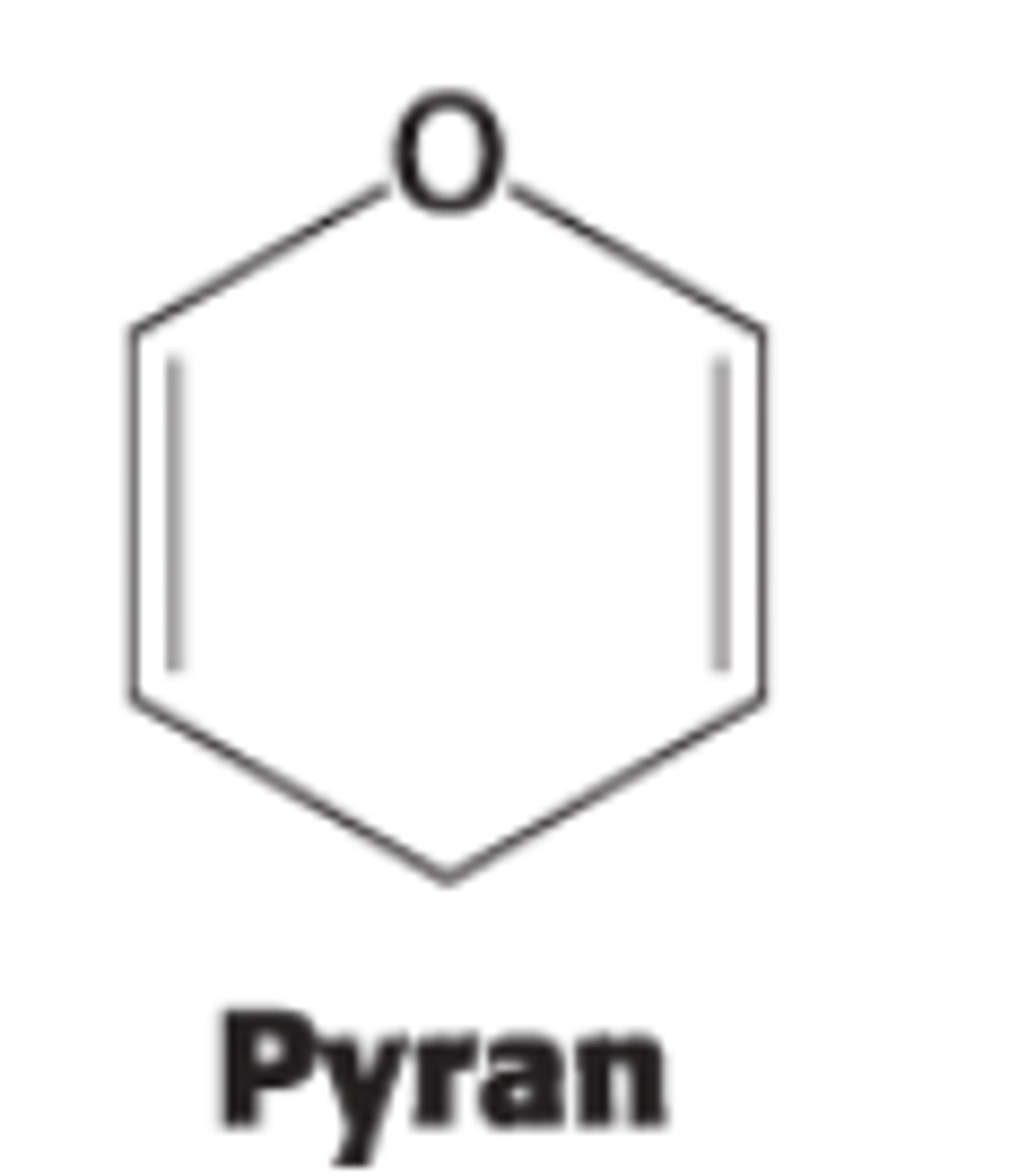
This resulting cyclic hemiacetal is called ?
pyranose due to its similarity to pyran (6 membered ring)
Define a hemiketal
ketone + alcohol = hemiketal
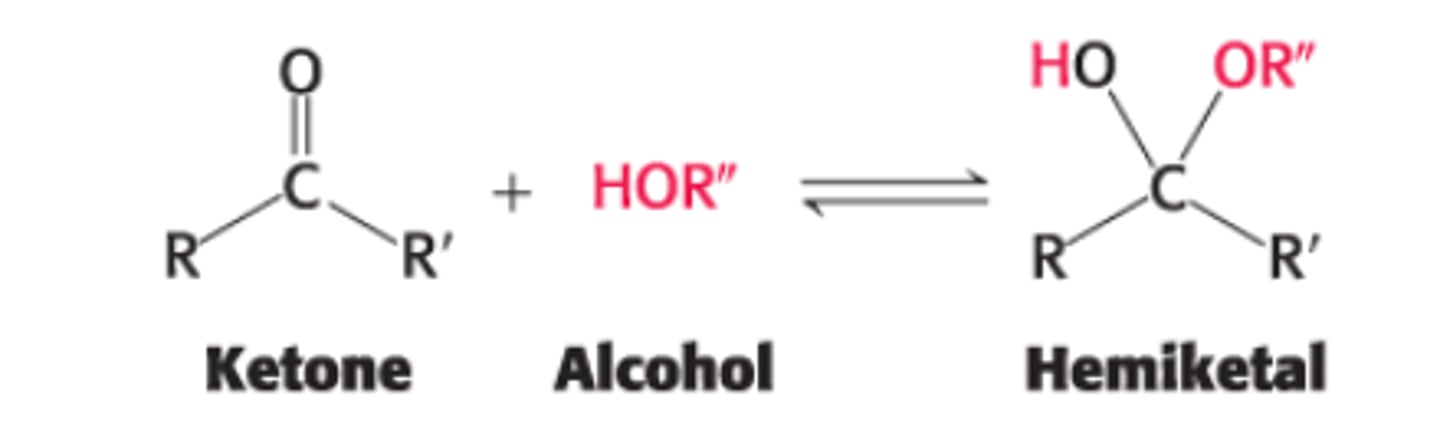
Explain the formation of an intramolecular hemiketal
C2 ketone group in open-chain form of a ketohexose such as fructose reacts with either its own C6 OH group (forming a 6 membered cyclic hemiketal) or C5 OH group (forming a 5 membered cyclic hemiketal)
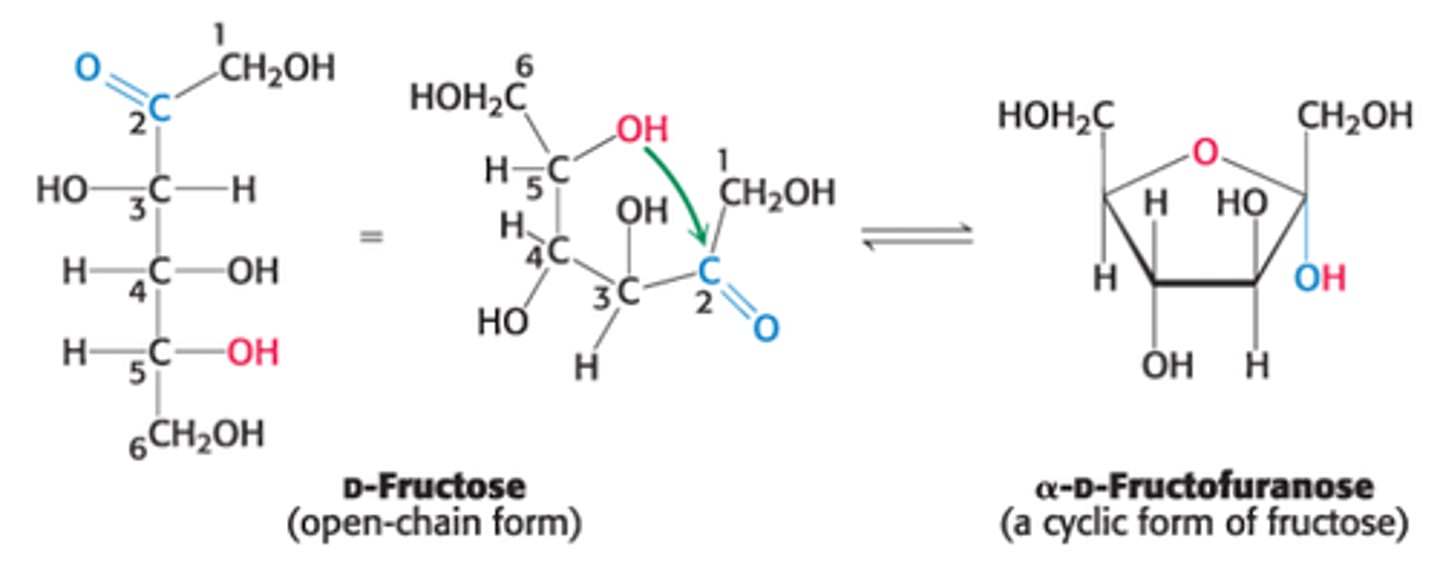
This 5 membered ring formed when C-5 OH group attacks the C2-ketone group in the open chain form of fructose is called ?
furanose due to its similarity to furan (5 membered ring)
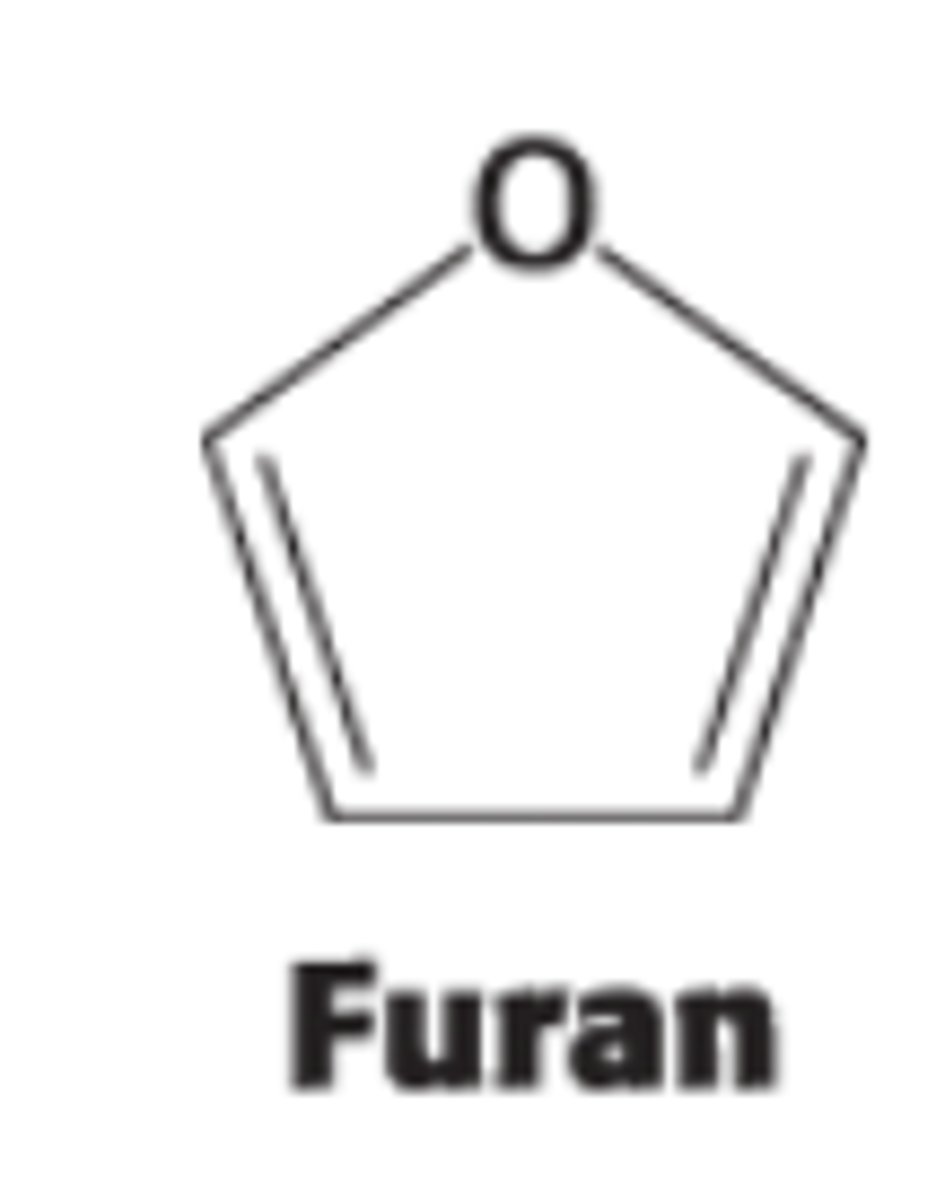
What 2 anomeric forms can result from furanose formation?
𝛼 & β but only 𝛼 is shown
Define an anomer
diastereoisomeric form of sugars that forms when a cyclic hemiacetal is created, resulting in an additional asymmetric center
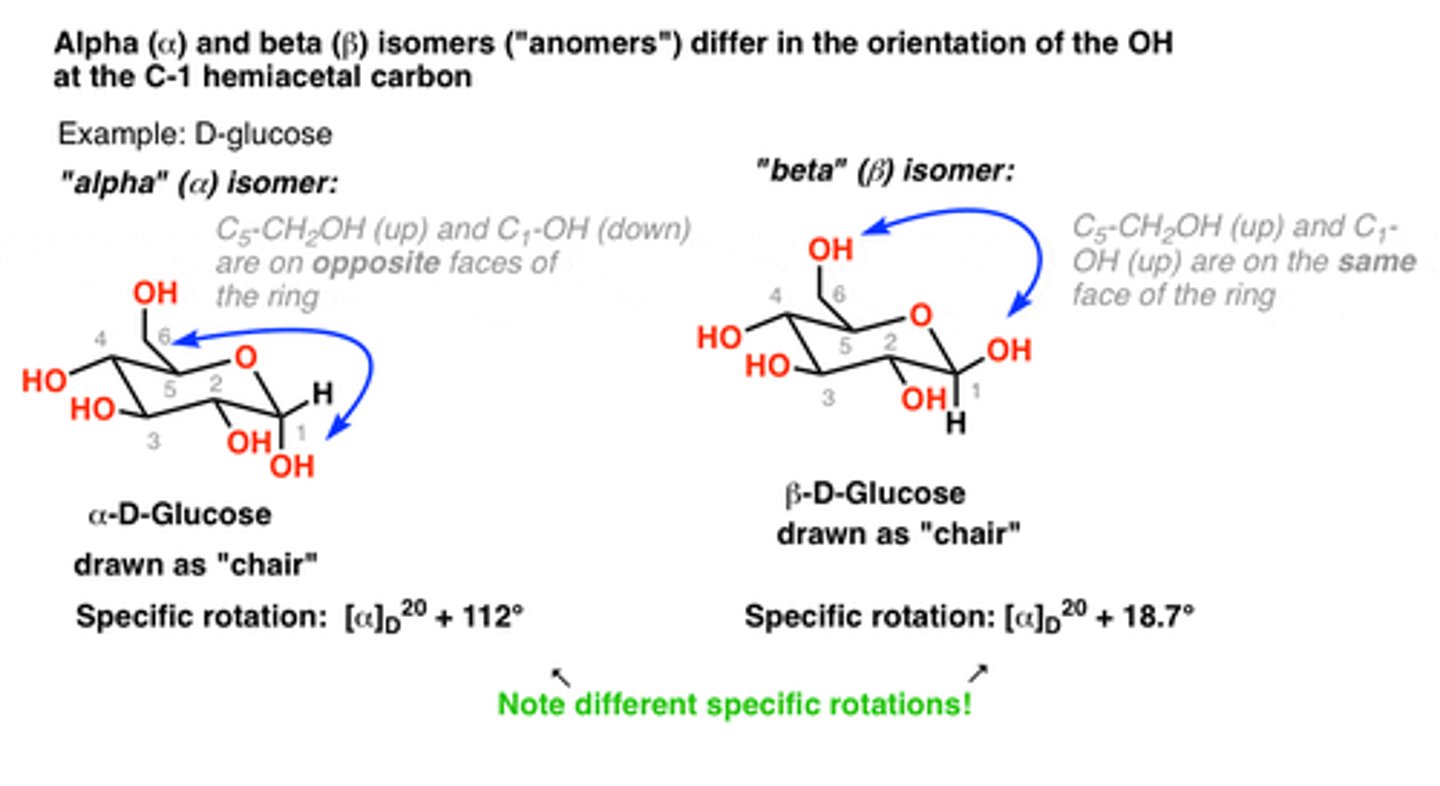
In glucose, which C atom is the anomeric C atom?
C1
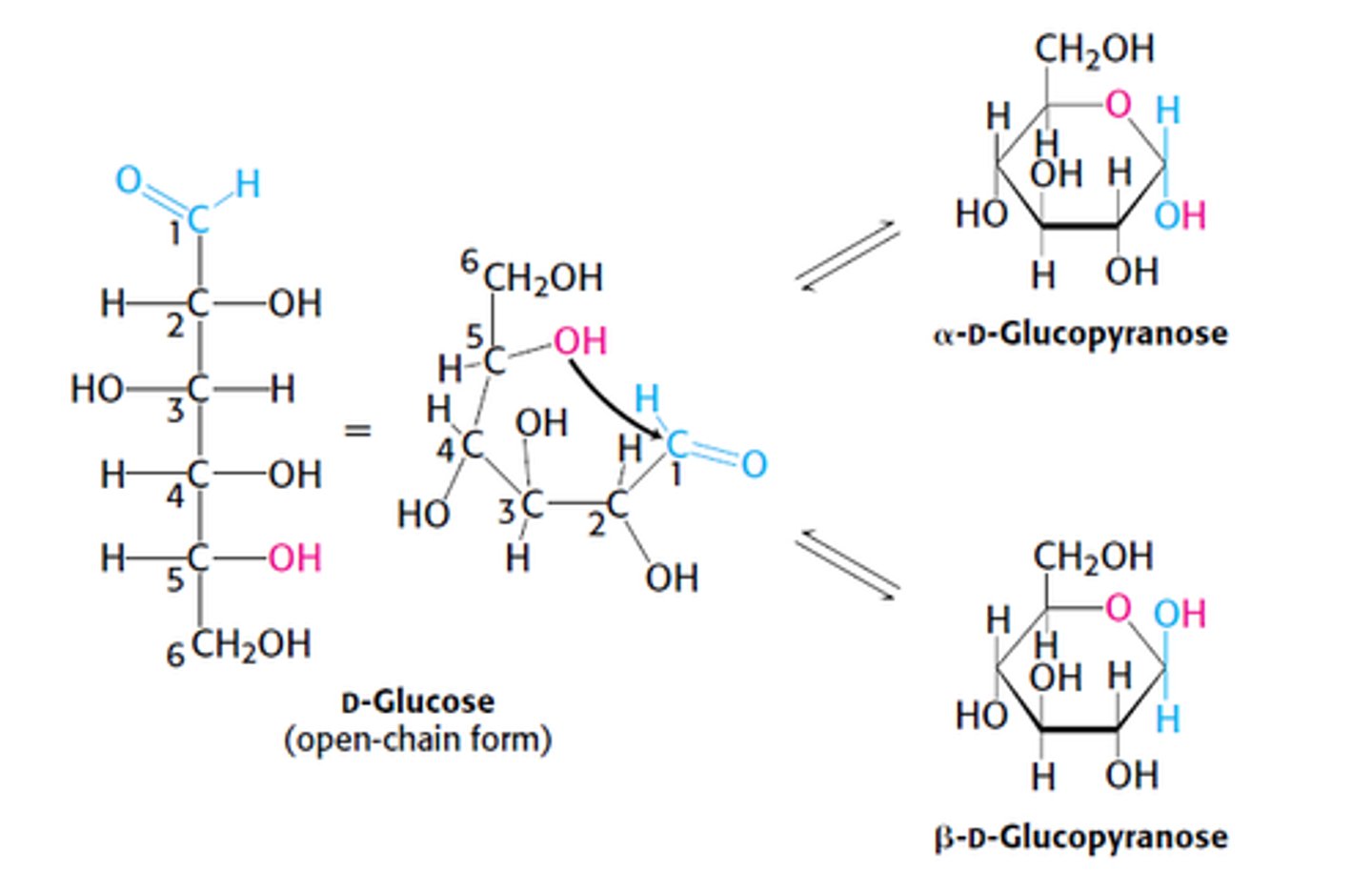
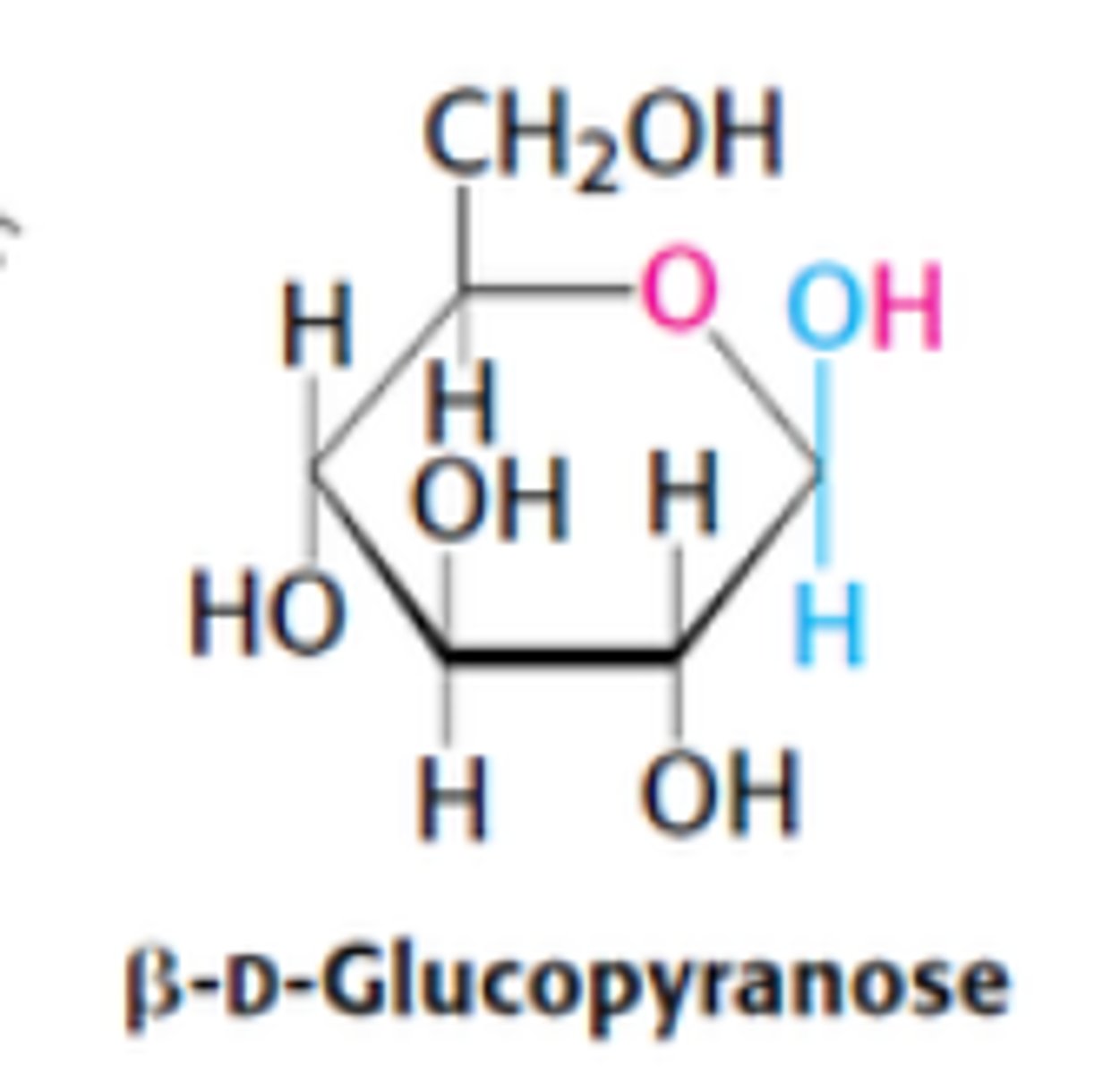
What are the two ring structures formed by glucose?
C1 becomes an asymmetric center resulting in 2 ring structures: α-D-glucopyranose and β-D-glucopyranose
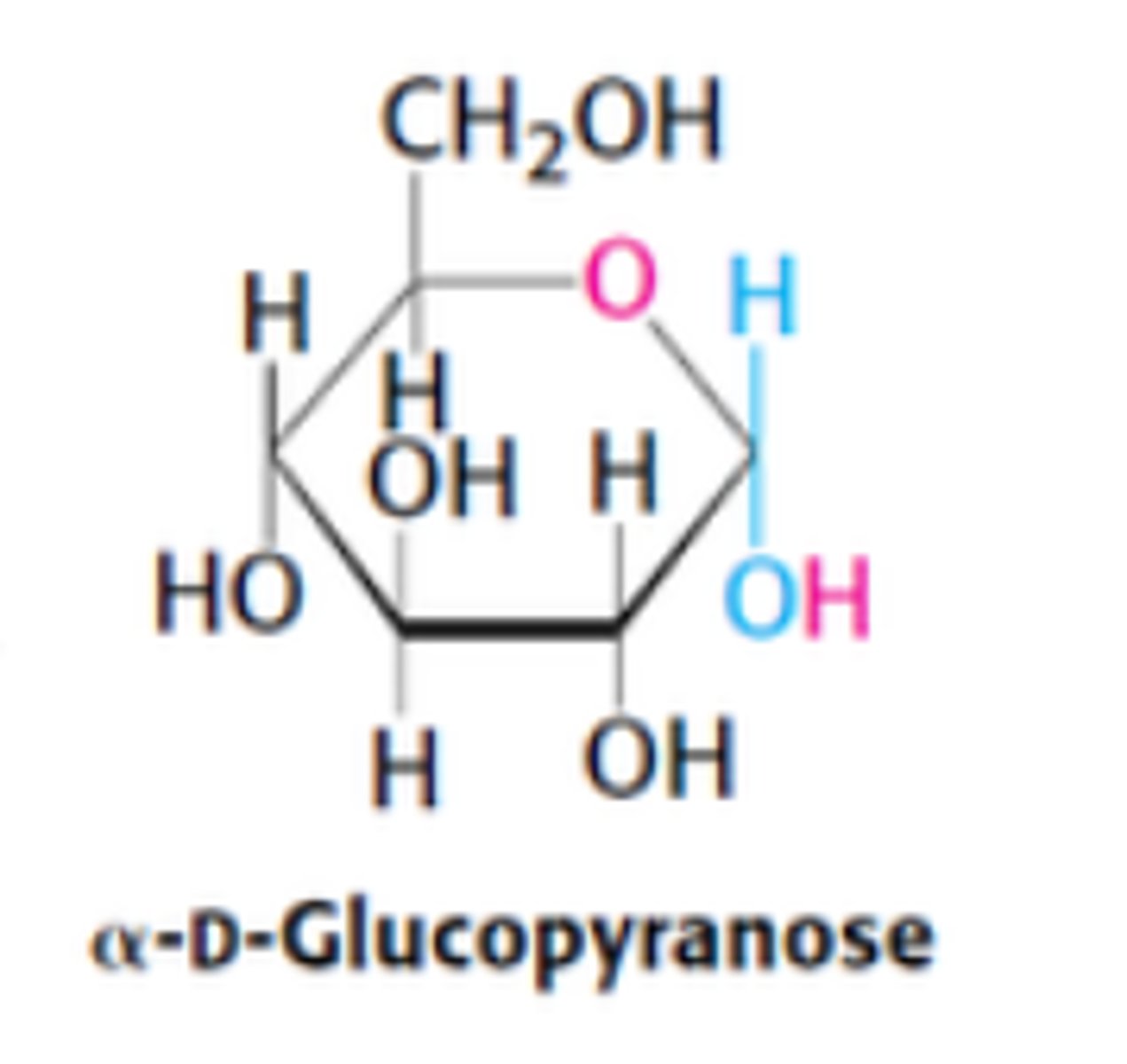
Define α-D-glucopyranose
OH group attached to C1 is on opposite side of ring as C6
Define β-D-glucopyranose
OH group attached to C1 is on same side of ring as C6
In D-Fructose (furanose ring form), which C is the anomeric/asymmetric C?
C2
Fructose forms which 2 types of rings?
1. Pyranose Ring - 6 membered ring
2. Furanose Ring - 5 membered ring
Fructose forms how many rings?
1. 2 furanoses (α & β forms)
2. 2 pyranoses (α & β forms)
*α & β - refer to the OH groups attached to C2, the anomeric C. Both in rapid equilibrium with the open chain form.
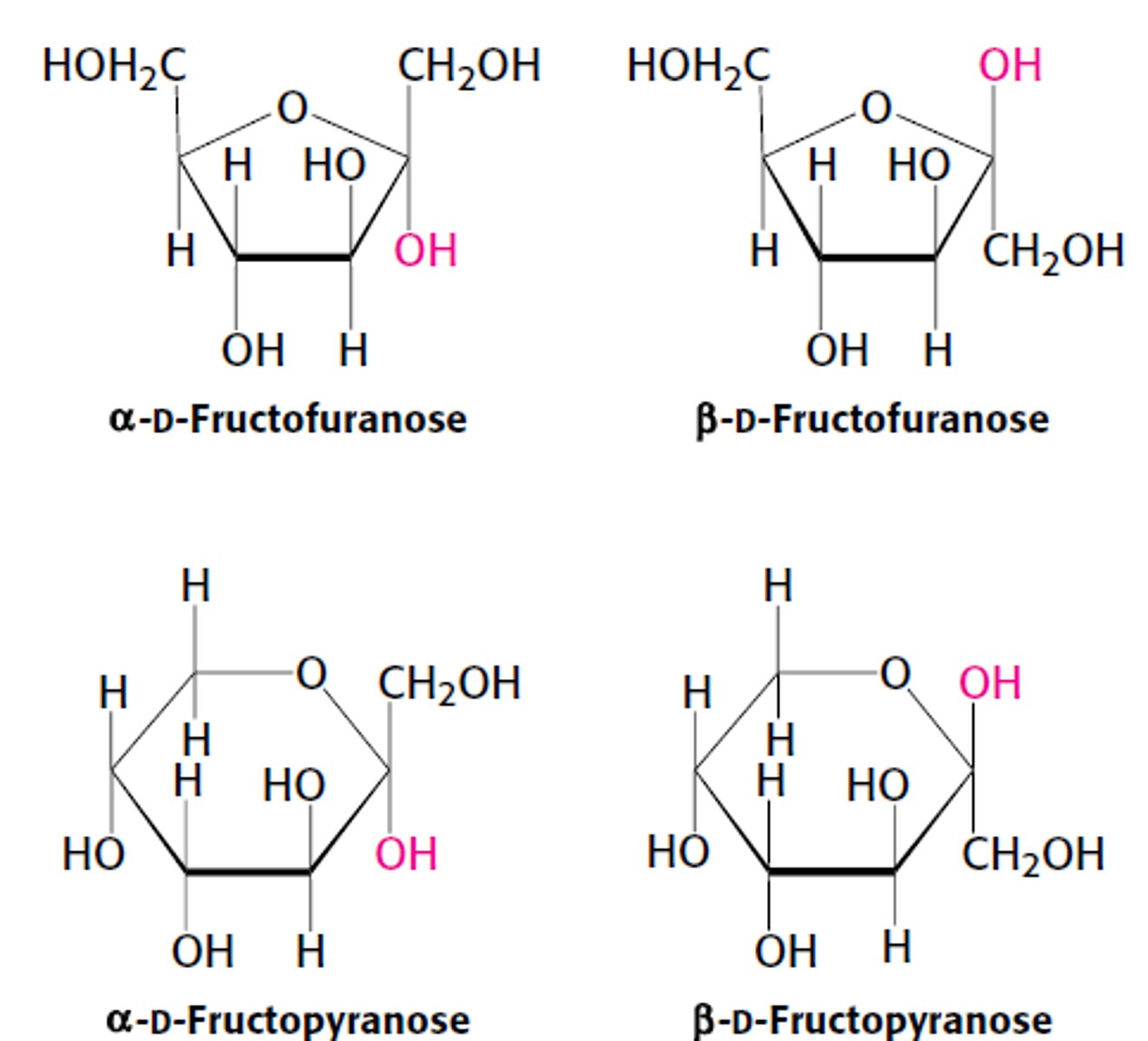
The pyranose form predominates in ?
fructose that is free in solution due to reduced steric hindrances
The furanose form predominates in ?
fructose derivatives
Summary of Isomeric Forms of Carbs

Pyranose rings are not planar due to
tetrahedral geometry of its saturated C atom
Instead, pyranose rings can adopt 2 types of conformations
1. Boat
2. Chair

Describe the chair form
substituents on C ring atoms have 2 orientations: axial or equatorial
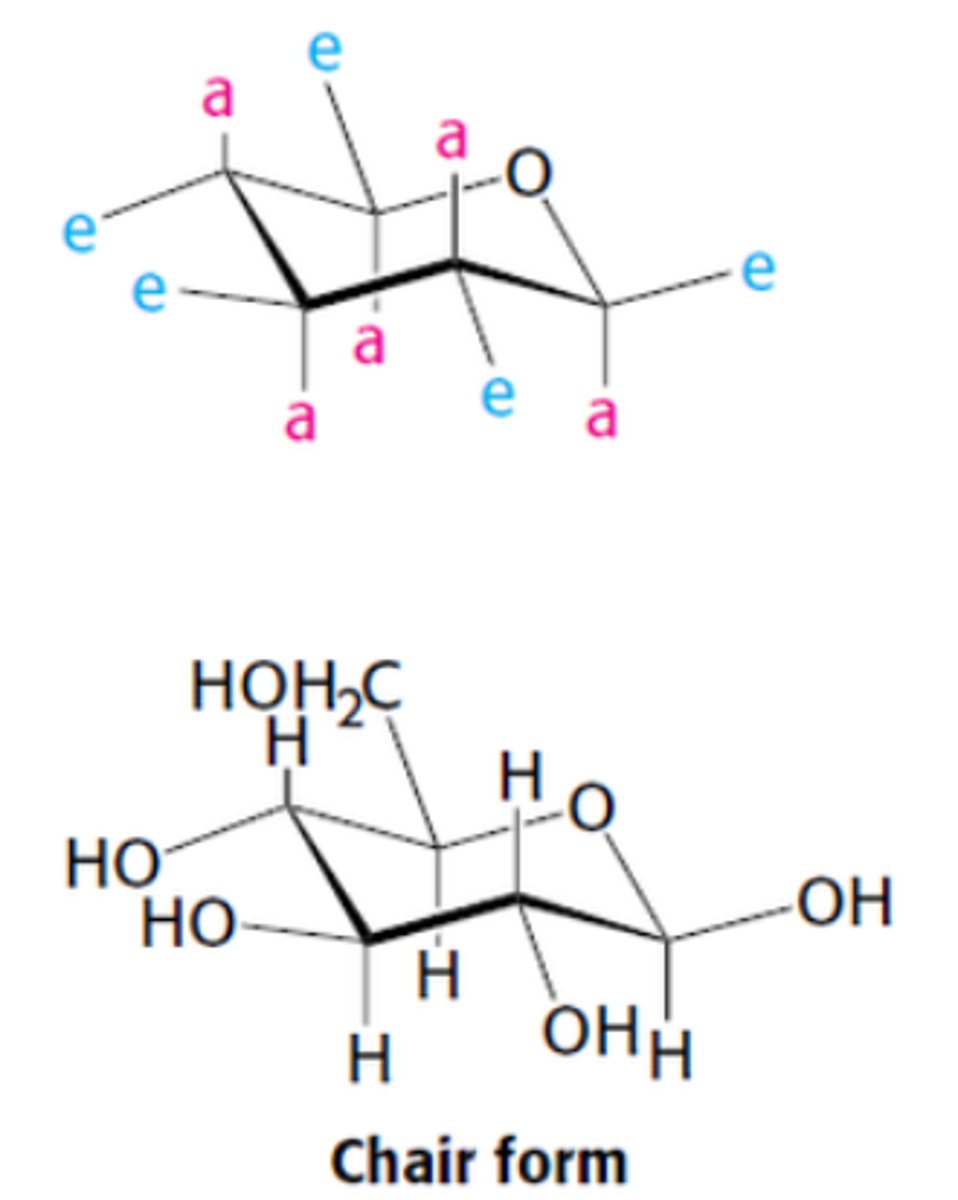
Define axial vs equatorial
Axial- nearly perpendicular to avg plane of the ring
Equatorial - nearly parallel to the plane
Axial substituents can ________ e/o if on same side of ring. In contrast, equatorial substituents are less ____.
sterically hinder, crowded
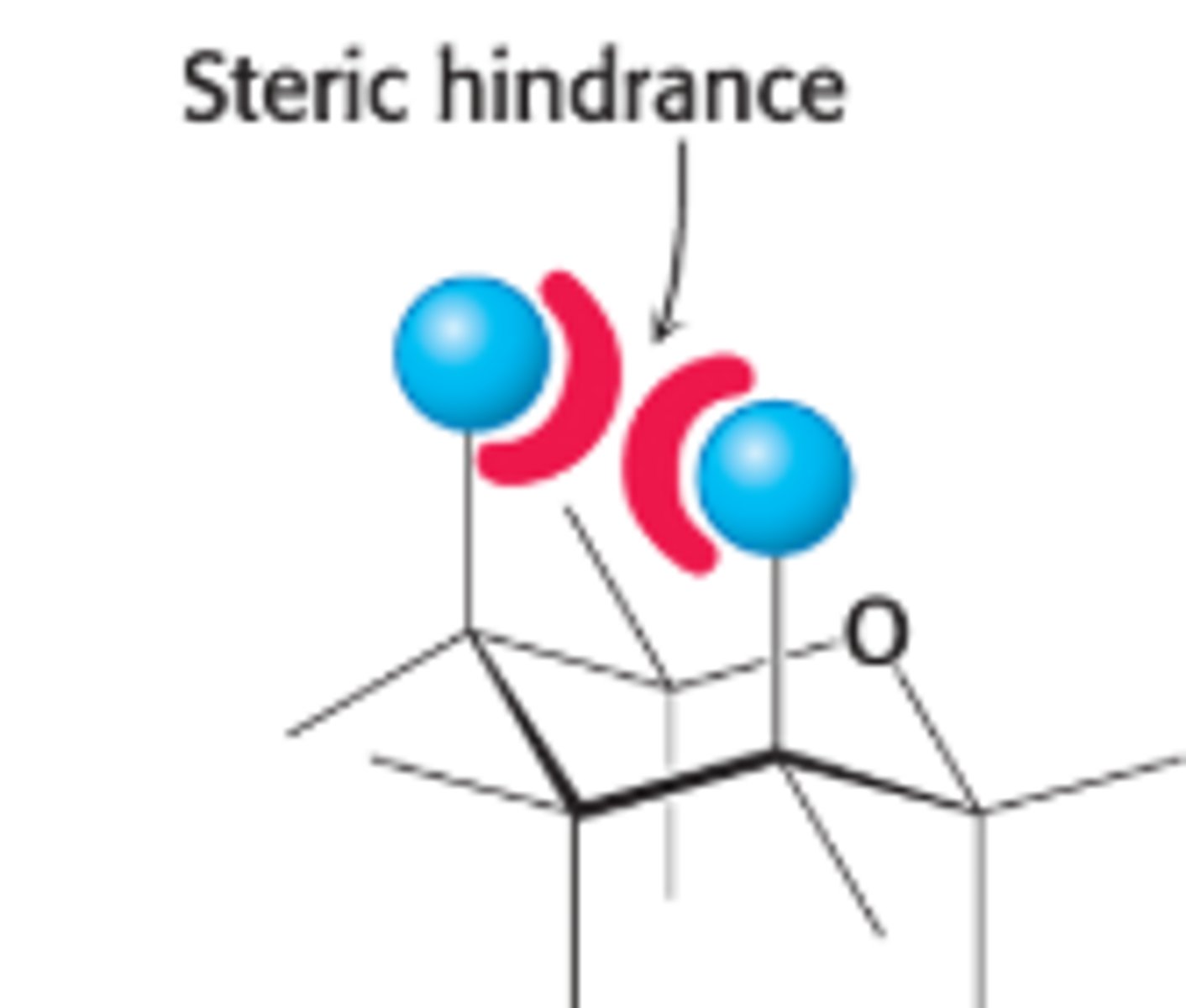
The chair form is more stable bc
H atoms occupy the axial positions, resulting in less steric hindrance unless in the boat form. The bulkier groups like OH & CH2OH emerges at less hindered perihery.
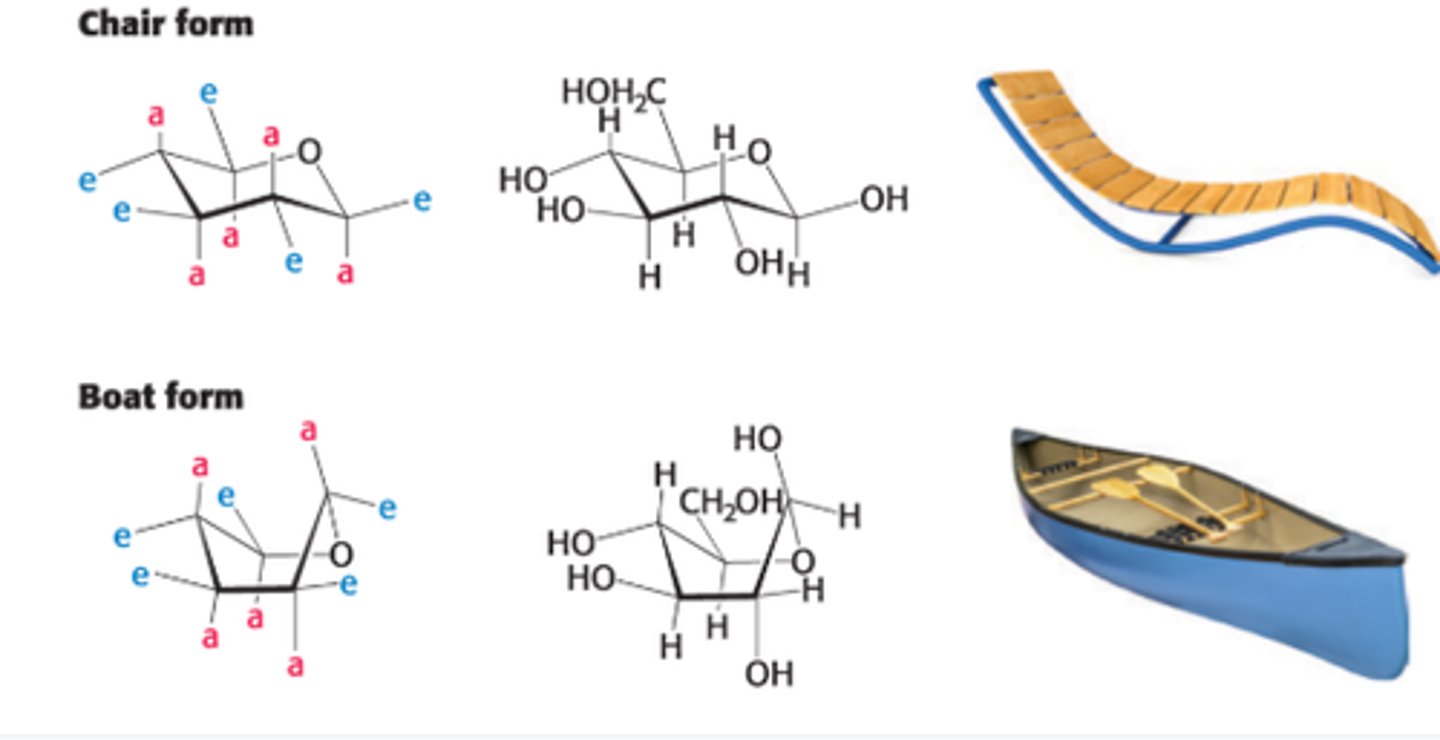
Why is the boat form of glucose disfavored?
due to steric hindrances
What's the importance of D-Glucose
important fuel for most organisms
Define blood sugar
D-glucose that circulates in blood
What is the significance of D-glucose in the body?
only fuel used by brain in non-starvation conditions + by RBC
What is glycation?
The nonenzymatic addition of a carbohydrate to another molecule.
What are some reasons D-glucose is an important fuel?
1. Glucose is formed from formaldehyde under prebiotic conditions and may have been available as a fuel source for primitive biochemical systems
2. Glucose is relatively inert/unstable
3. Most stable ring structure is β-D-glucopyranose
Define reducing vs non reducing sugars
Reducing Sugars - sugars that react or open up into a form that contains a reactive aldehyde group that can react with a free amino group (NH2) on a protein
Nonreducing Sugars - sugars that do NOT react
Reducing sugars often nonspecifically react with
free AA groups (amino groups) on Lys/Arg proteins to form a (temp cov bond first then) stable covalent bond
Lys - amino group in side chain
Arg - reactive N group
Define Glycation/Glycosylation
nonenzymatic addition of a carb (such as a sugar molecule) to another molecule such as protein or lipid
Does D-glucose have a high or low tendency to glycate proteins?
Low tendency to glycate proteins since it usually stays in its cyclic (ring) form not its reactive open-form that would open up an aldehyde group
D-glucose' low tendency to glycate proteins can be overcome by
high concentrations of sugar and protein for long periods of time
Define advanced glycation end products (AGEs)
Once the initial glucose-protein bond is formed, it can undergo further complex rxns such as cross-linking. The end products after cross-linking are called AGEs.
AGEs cause _____ b/w proteins
cross-links between other proteins making them more rigid and less flexible
Why are AGEs often detrimental? What are they involved in?
They are often detrimental bc they alter normal biochemical function of the modified proteins. These modifications (AGEs) are involved in aging, arteriosclerosis, diabetes and other conditions
As a reducing sugar, D-glucose reacts with _____ to form _________ Hb
reacts with Hb/glycates it to form glycated or glycosylated Hb
What's another word for glycated Hb?
Hb A1C - measures how much Hb has been glycated
Does this have an effect on O2 binding
No
Monitoring changes in amount of ______ is a useful means of assessing effectiveness of treatments for diabetes mellitus
glycosylated Hb. Since glycosylated Hb remains in circulation, the amount of modified Hb corresponds to long-term regulation (over 6 months of glucose levels)
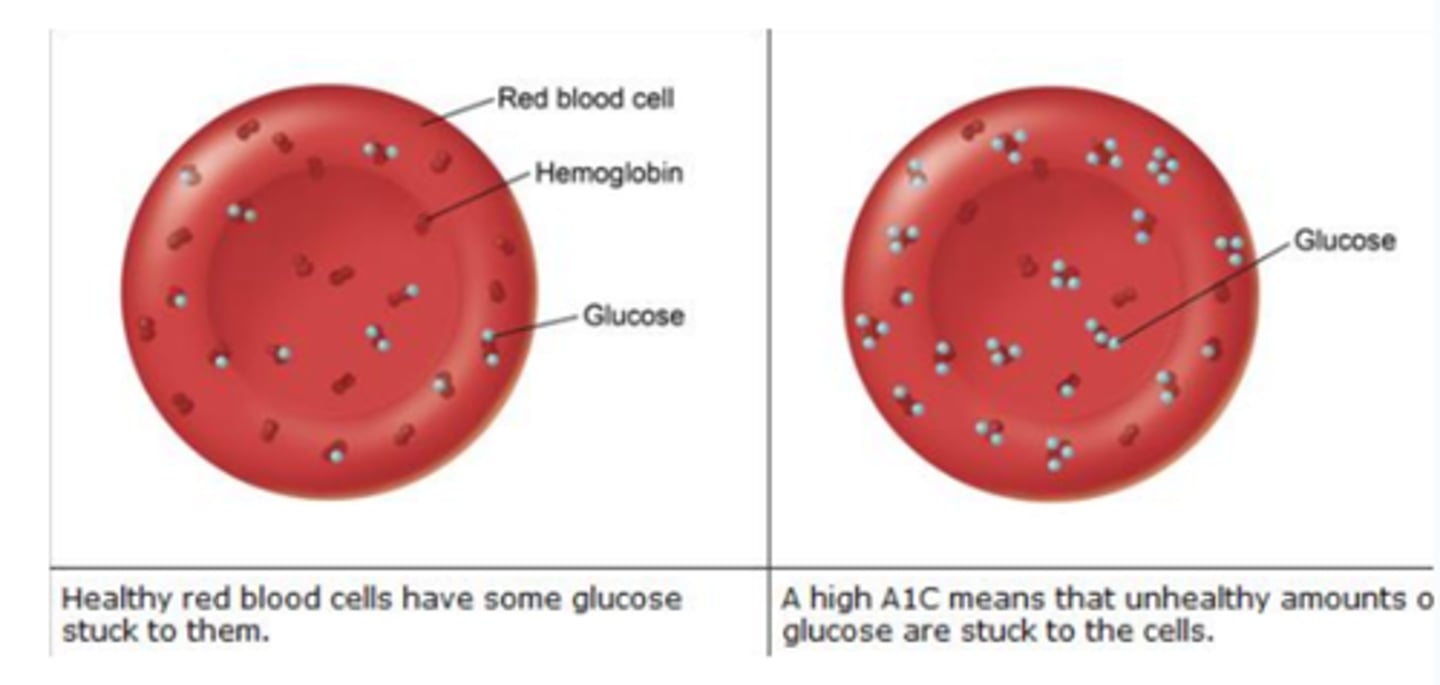
In nondiabetic individuals, less than ___% of Hb is glycosylated
6%
In patients with uncontrolled diabetes, almost __% of Hb is glycated
10%
What is the lifespan of red blood cells, affecting A1C levels?
120 days
The biochemical properties of a monosaccharide can be modified by reactions with 3 types of molecules
1. Alcohols
2. Amines (NH3)
3. Phosphates
These modifications of monosaccharides increase _______ ________
biochemical versatility, enabling them to now serve as signaling molecules or facilitate metabolism
Define a glycosidic linkage/bond
covalent linkage b/w anomeric/asymmetric C of a carb + O atom of an alcohol or N atom of an amine
Glycosidic bonds are prominent when
carbs are linked together to form long polymers & when a carb is attached to proteins
Since sugars contain OH grous, _______ bonds can join 1 monosaccharide to another
glycosidic
Define an O-glycosidic linkage
covalent bond formed b/w anomeric C atom of a carb such as glucose & O atom of an alcohol/OH group
Ex: O-glycosidic bond linking glucose to a methyl group

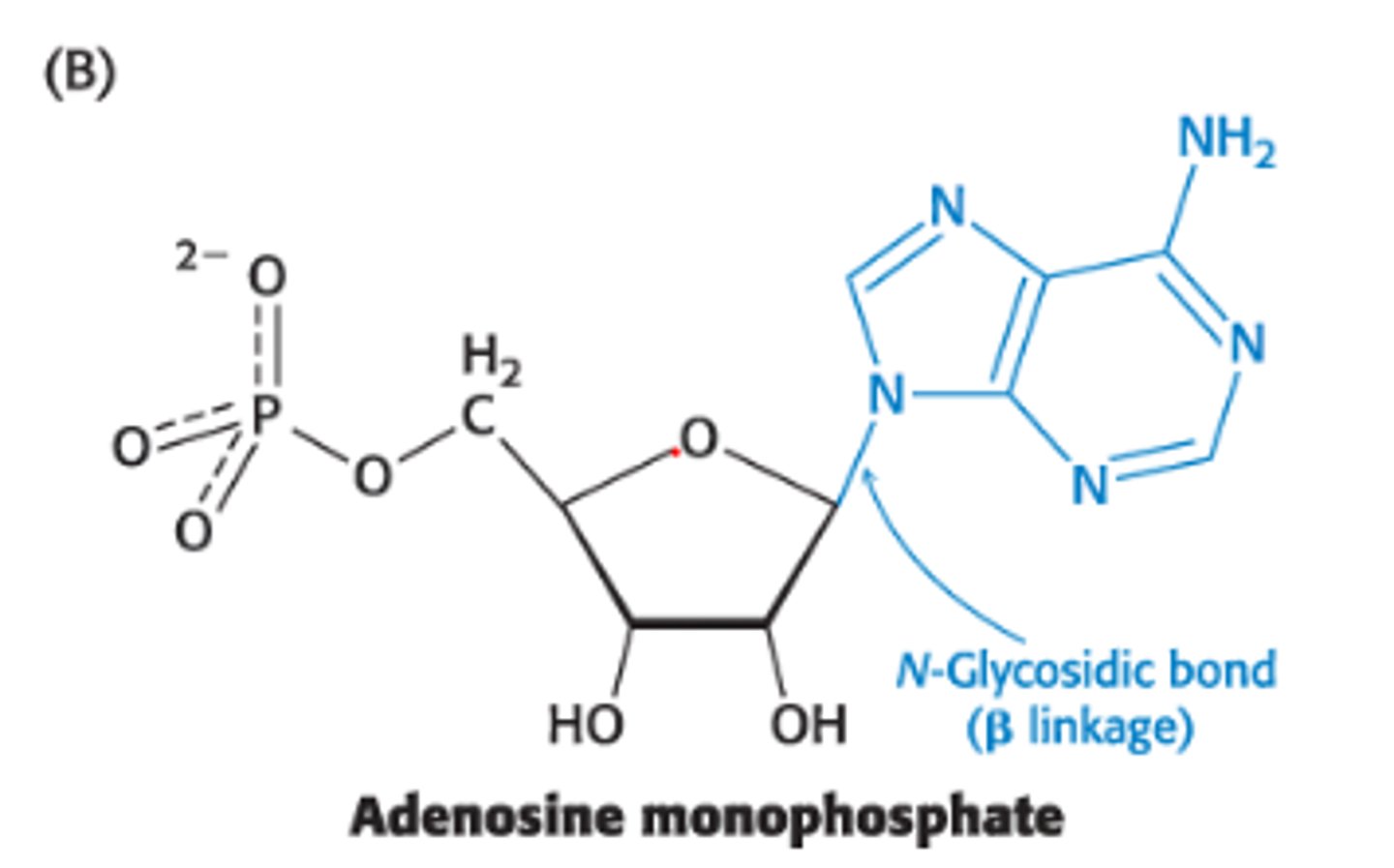
Define an N-glycosidic linkage?
covalent linkage b/w the anomeric/asymmetric C atom of a carb + N atom of an amine
Ex: Nitrogenous bases attached to Ribose units to form nucleosides via N-glycosidic linkages
Carbs can also be modified by attachment of _______ groups to Cs other than OH groups at the anomeric/asymmetric C
functional
Define phosphorylation
common modification of sugars in metabolic reactions via attachment of phosphoryl groups
What's the charge on sugars after phosphorylation?
anionic = negative charge
What's the role of phosphorylated sugars?
1. prevents sugars from crossing the lipid-bilayer membranes spontaneously and interacting with transporters of the unmodified sugar
2. blocks the formation of alternative ring conformation
3. creates rxn intermediates that more readily undergo metabolism
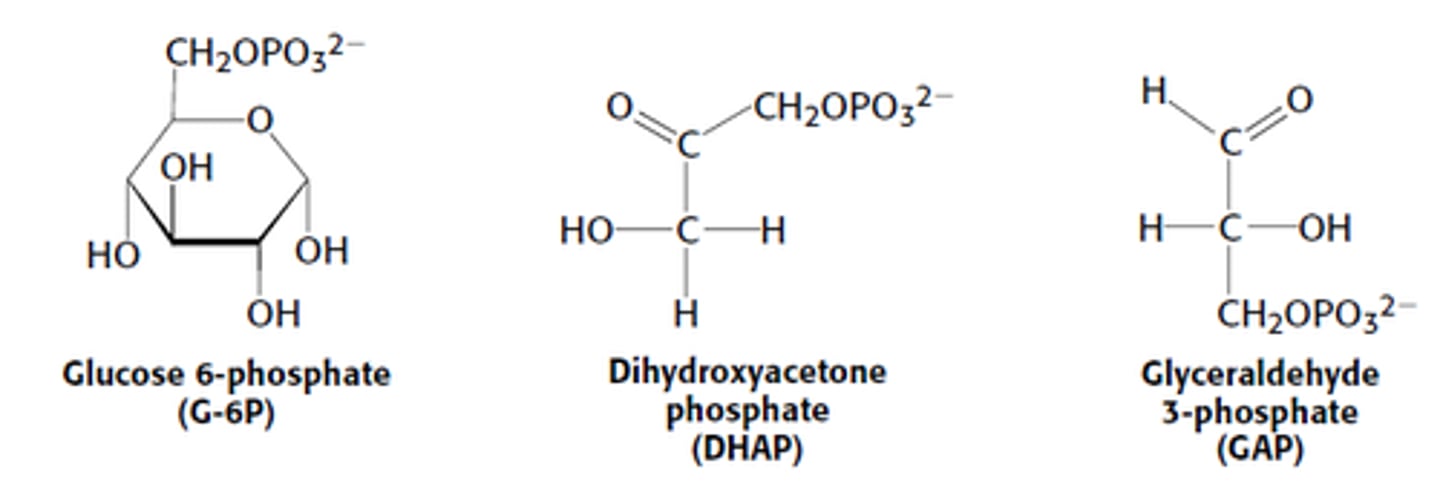
The first step in breakdown of glucose to obtain energy is
glucose' conversion into glucose-6-phosphate. Other intermediates in this path include G-6PP, DHAP and GAP.
Define oligosaccharides
two or more monosaccharides/sugars linked by O-glycosidic bonds (
What defines the directionality of oligosaccharides?
Their reducing and nonreducing ends NOT reducing sugars
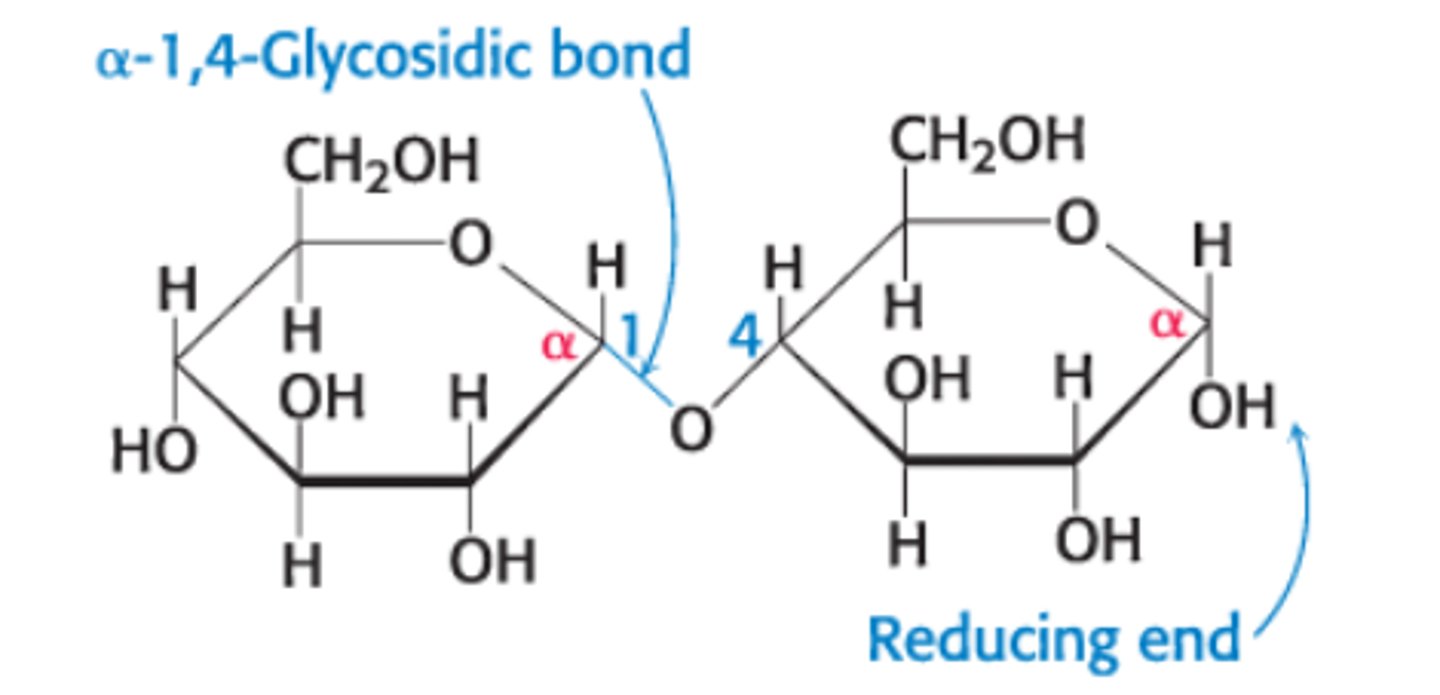
Define reducing vs nonreducing end
Reducing End - free anomeric C atom that can form the open-chain form
Nonreducing End - anomeric C in glyosidic linkage that cannot convert to the open-chain form
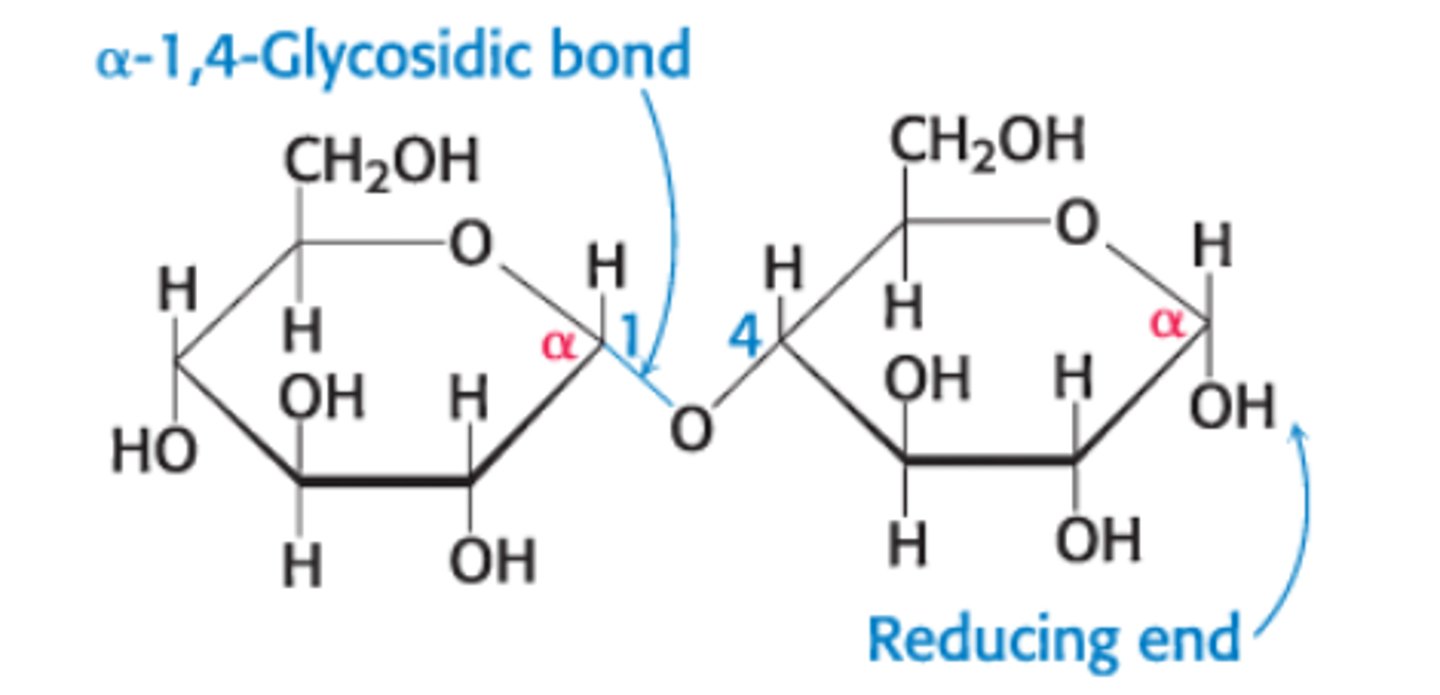
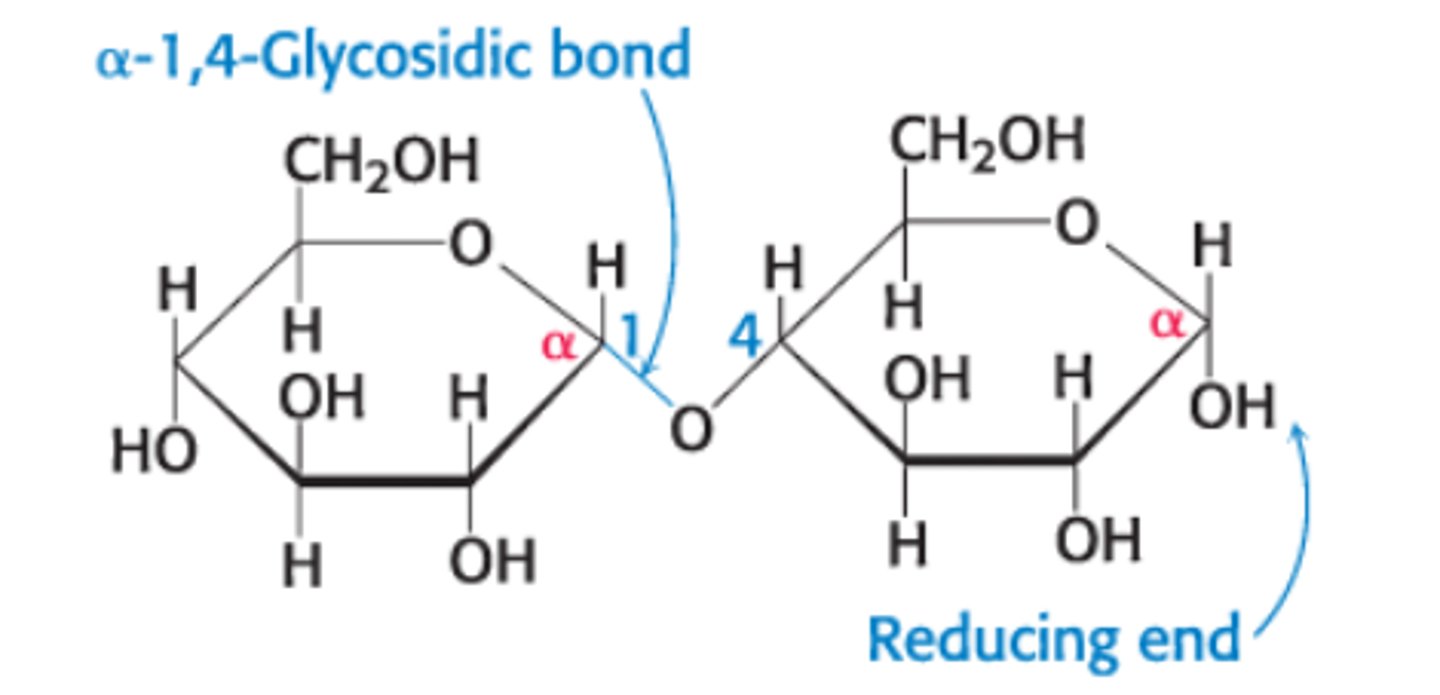
Define an α-1,4-glycosidic linkage
glycosidic linkage between the α-anomeric form of C-1 on one sugar and the O atom of OH group on C-4 of the adjacent sugar
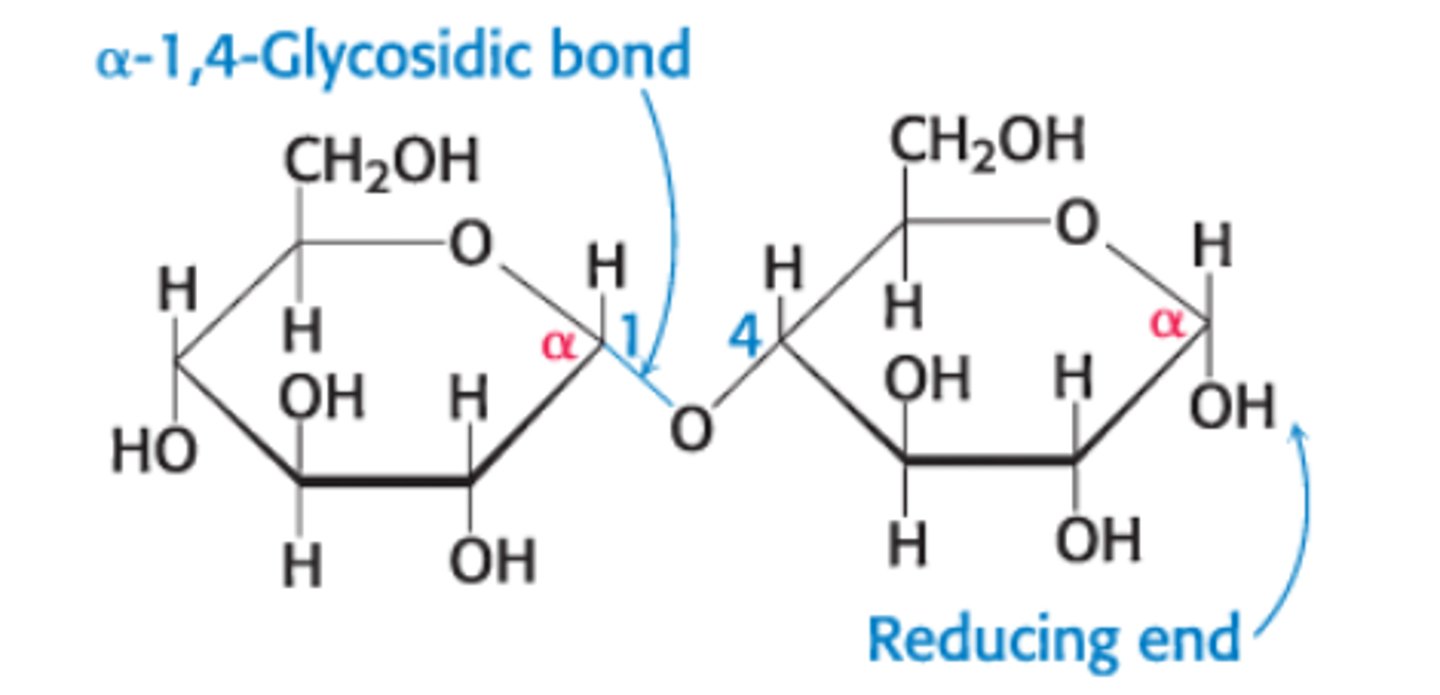
What does α vs β refer to?
α - OH on the anomeric C pointing down
β - OH on anomeric C pointing up
What is maltose?
A disaccharide composed of glucose + glucose molecules linked by an α-1,4-glycosidic linkage
Glucose molecule on RT: can assume open-chain form which can act as a reducing agent/oxidize other molecules since it has a free anomeric C. It's still called reducing even when it's bound to another molecule such a a protein and may not longer have reducing properties.
Glucose molecule on LT: CANNOT assume open-chain form since the C1 is bound to another molecule
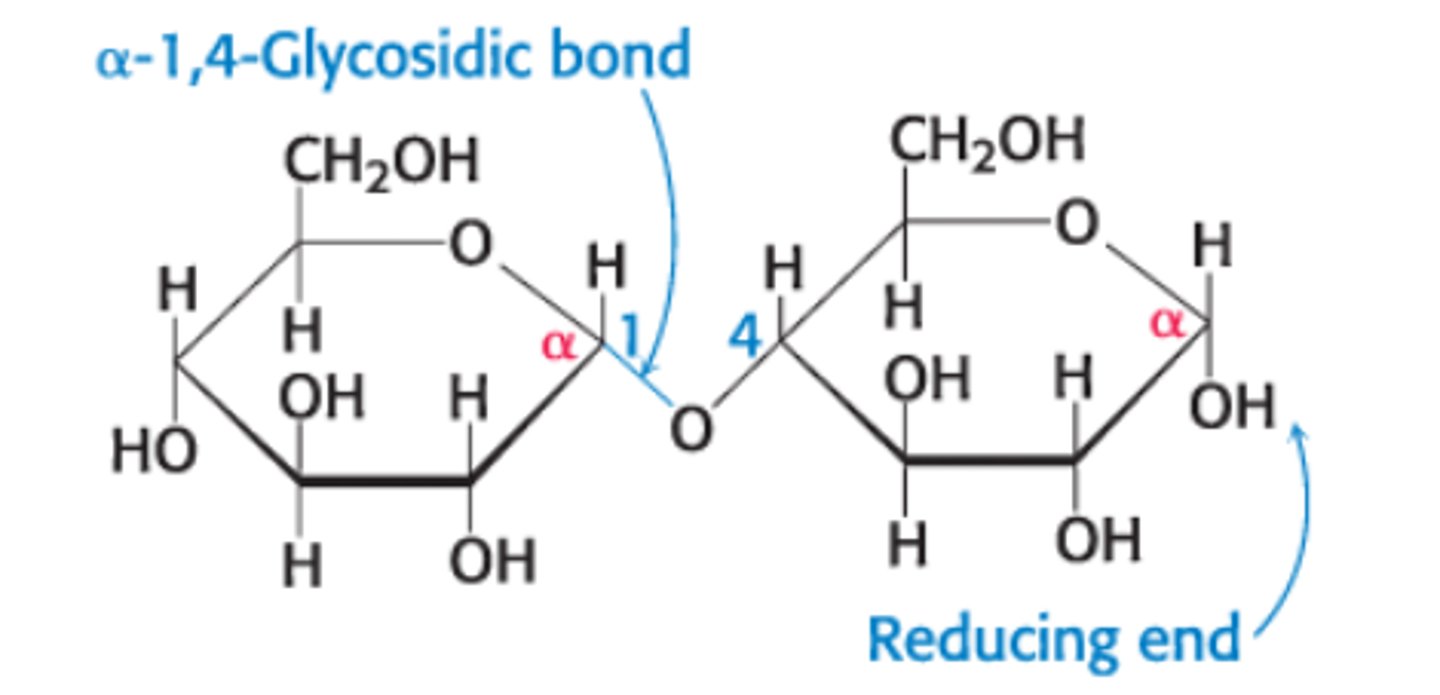
Maltose is a _____ sugar
reducing sugar since it has a free anomeric carbon on one of its two glucose units, which can open to form an aldehyde group
The fact that monosaccharides have multiple OH groups means
that many diff glycosidic linkages can be formed since these bonds join monosaccharides together via OH groups
What are the common disaccharides?
Sucrose, lactose, and maltose.
Define a disaccharide
2 sugars joined by O-glycosidic bond
Cleavage products of disaccharides can be processed to provide energy in the form of
ATP
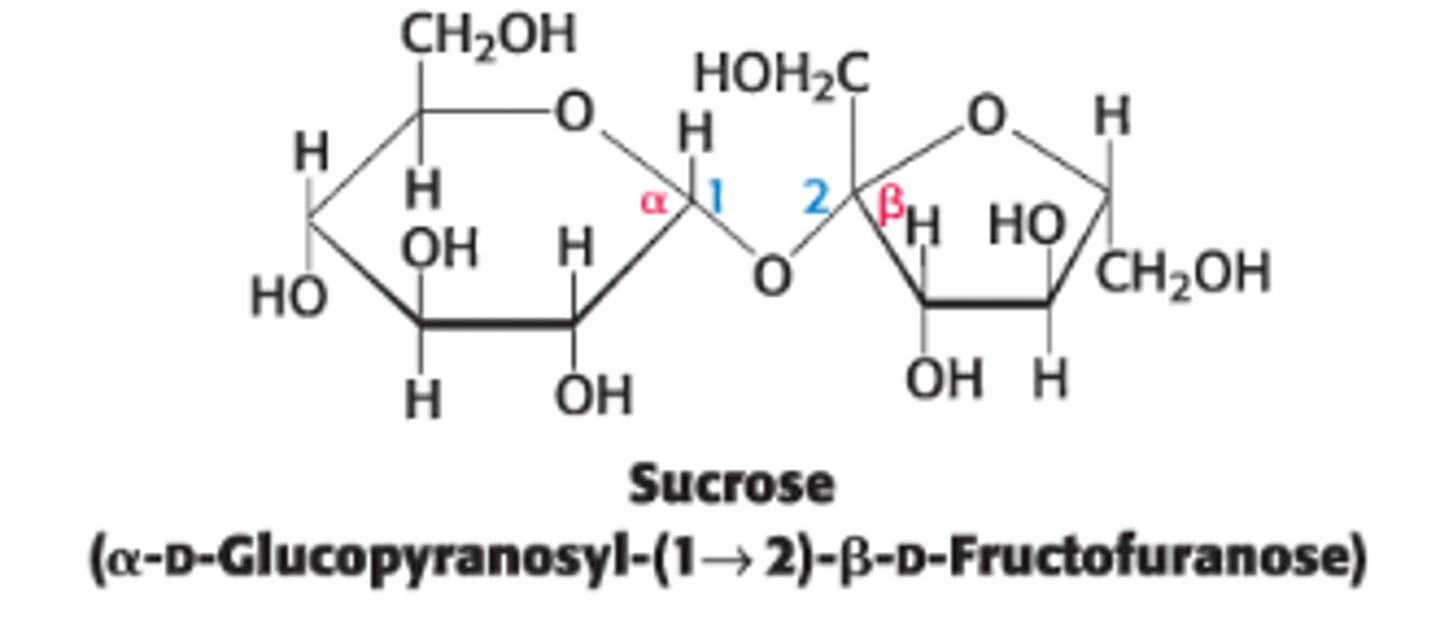
Define sucrose & its components
disaccharide of sugar cane or sugar beets, consisting of glucose (6 membered ring) + fructose (5 membered ring)
What type of linkage connects fructose to glucose in sucrose?
anomeric carbon of glucose is linked to the anomeric carbon of fructose
What type of sugar is sucrose classified as?
Sucrose is not a reducing sugar
Why is sucrose not a reducing sugar?
neither sugar can access the open chain form since both anomeric C's are involved in the glycosidic linkage
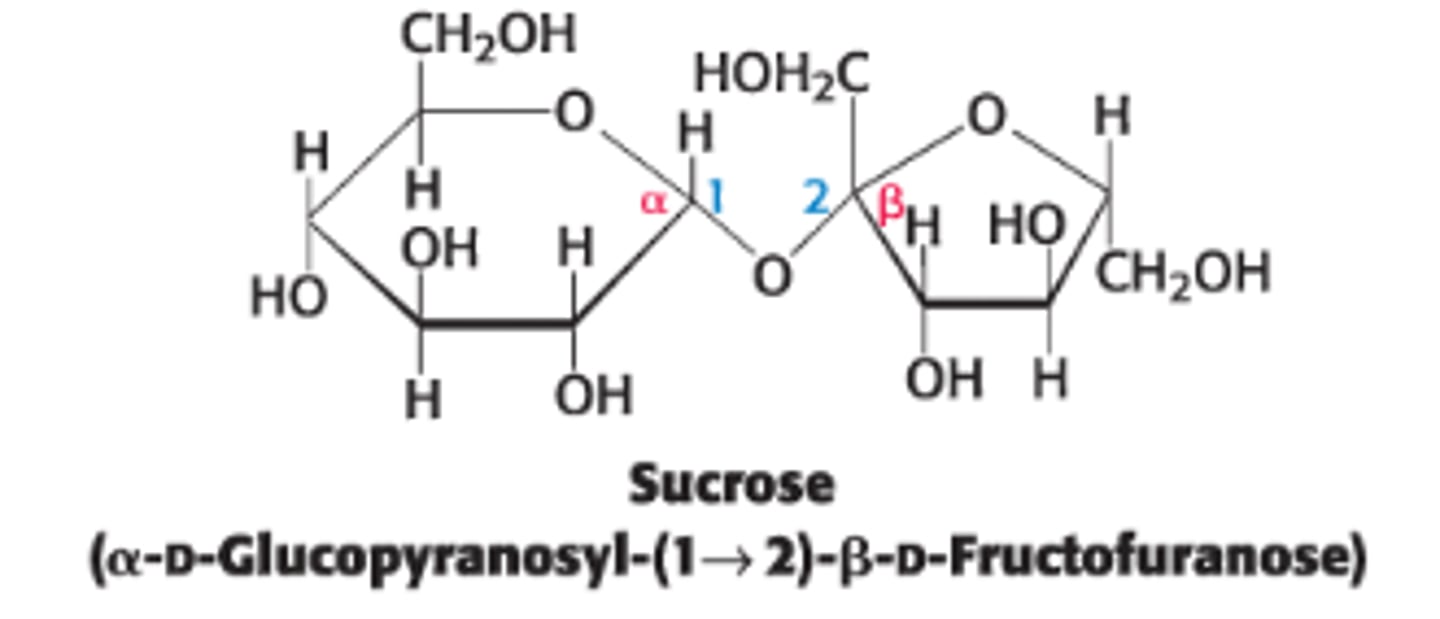
Doese sucrose have anomeric ends?
No
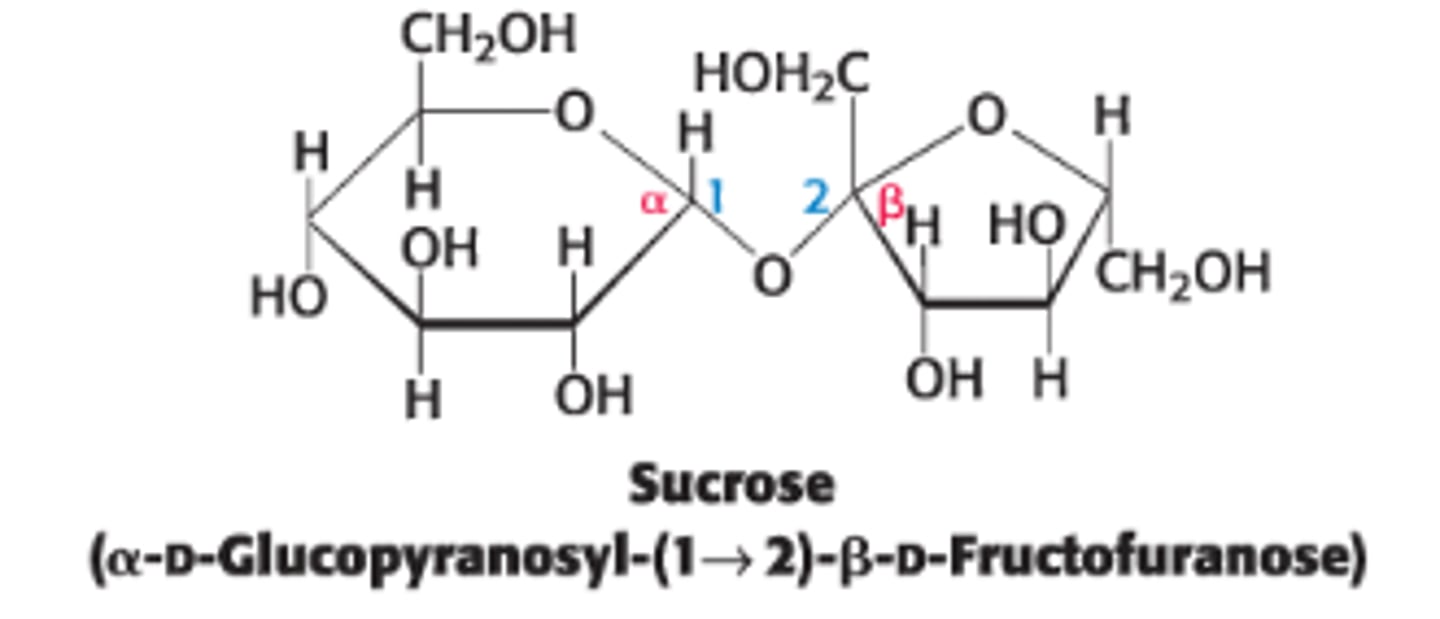
What's the configuration of glucose and fructose in sucrose?
α for glucose and β for fructose
How is sucrose hydrolyzed?
Sucrose can be cleaved by the enzyme sucrase (invertase).
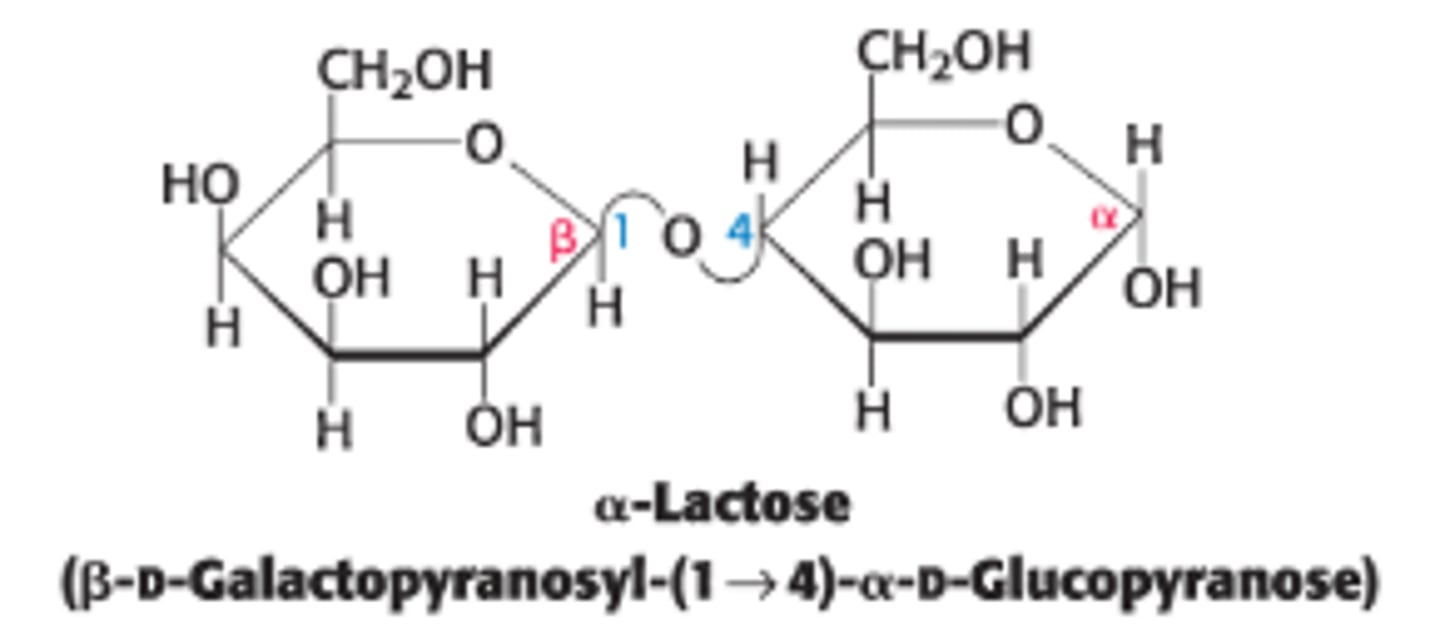
Define lactose and its components
a disaccharide of milk that consists of galactose + glucose
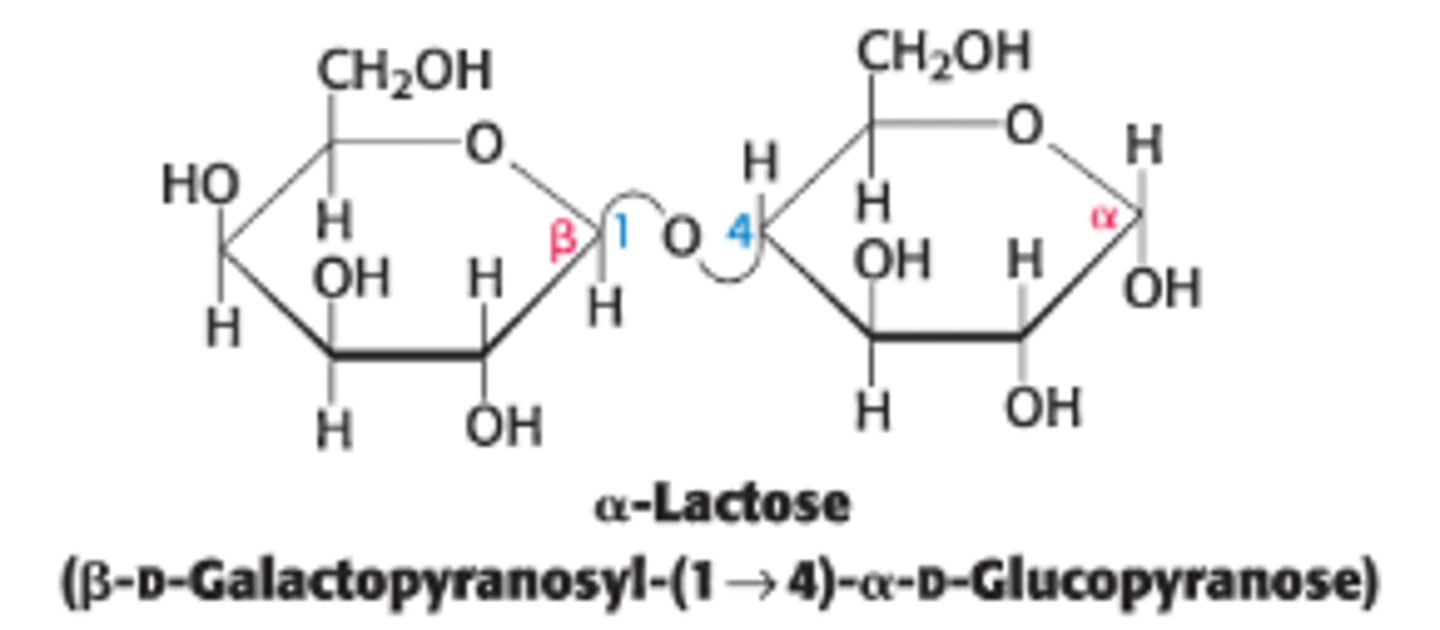
What type of linkage connects galactose to glucose in lactose?
by a β-1,4-glycosidic linkage - 1st sugar has a β-anomeric carbon at C1 (β - OH pointing up) that bonds to the C4-OH group on the 2nd sugar